Abstract
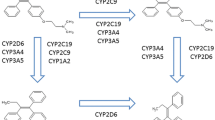
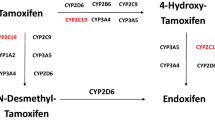
CYP2D6 is a key enzyme responsible for the metabolism of tamoxifen to active metabolites, endoxifen, and 4-hydroxytamoxifen. The breast cancer patients who are heterozygous and homozygous for decreased-function and null alleles of CYP2D6 showed lower plasma concentrations of endoxifen and 4-hydroxytamoxifen compared to patients with homozygous-wild-type allele, resulting in worse clinical outcome in tamoxifen therapy. We recruited 98 Japanese breast cancer patients, who had been taking 20 mg of tamoxifen daily as adjuvant setting. For the patients who have one or no normal allele of CYP2D6, dosages of tamoxifen were increased to 30 and 40 mg/day, respectively. The plasma concentrations of tamoxifen and its metabolites were measured at 8 weeks after dose-adjustment using liquid chromatography–tandem mass spectrometry. Association between tamoxifen dose and the incidence of adverse events during the tamoxifen treatment was investigated. In the patients with CYP2D6*1/*10 and CYP2D6*10/*10, the mean plasma endoxifen levels after dose increase were 1.4- and 1.7-fold higher, respectively, than those before the increase (P < 0.001). These plasma concentrations of endoxifen achieved similar level of those in the CYP2D6*1/*1 patients receiving 20 mg/day of tamoxifen. Plasma 4-hydroxytamoxifen concentrations in the patients with CYP2D6*1/*10 and CYP2D6*10/*10 were also significantly increased to the similar levels of the CYP2D6*1/*1 patients according to the increasing tamoxifen dosages (P < 0.001). The incidence of adverse events was not significantly different between before and after dose adjustment. This study provides the evidence that dose adjustment is useful for the patients carrying CYP2D6*10 allele to maintain the effective endoxifen level.




Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351:1451–1467
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Borgna JL, Rochefort H (1981) Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem 256:859–868
Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM (1989) Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res 49:2175–2183
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159
Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, Skaar TC (2006) Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther 318:503–512
Lim HS, Lee JH, Lee SK, Lee SE, Jang IJ, Ro J (2007) Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 25:3837–3845
Jin Y, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310:1062–1075
Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM (2002) Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4’-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30:869–874
Broly F, Gaedigk A, Heim M, Eichelbaum M, Morike K, Meyer UA (1991) Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol 10:545–558
Sachse C, Brockmoller J, Hildebrand M, Muller K, Roots I (1998) Correctness of prediction of the CYP2D6 phenotype confirmed by genotyping 47 intermediate and poor metabolizers of debrisoquine. Pharmacogenetics 8:181–185
Nakamura K, Goto F, Ray WA, McAllister CB, Jacqz E, Wilkinson GR, Branch RA (1985) Interethnic differences in genetic polymorphism of debrisoquin and mephenytoin hydroxylation between Japanese and Caucasian populations. Clin Pharmacol Ther 38:402–408
Yokota H, Tamura S, Furuya H, Kimura S, Watanabe M, Kanazawa I, Kondo I, Gonzalez FJ (1993) Evidence for a new variant CYP2D6 allele CYP2D6 J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics 3:256–263
Kiyotani K, Mushiroda T, Sasa M, Bando Y, Sumitomo I, Hosono N, Kubo M, Nakamura Y, Zembutsu H (2008) Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci 99:995–999
Goetz MP, Rae JM, Suman VJ et al (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318
Goetz MP, Knox SK, Suman VJ et al (2007) The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101:113–121
Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM, Simon W, Eichelbaum M, Brauch H (2007) Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol 25:5187–5193
Xu Y, Sun Y, Yao L et al (2008) Association between CYP2D6*10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol 19:1423–1429
Schroth W, Goetz MP, Hamann U et al (2009) Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302:1429–1436
Kiyotani K, Mushiroda T, Imamura CK et al (2010) Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol 28:1287–1293
Gjerde J, Geisler J, Lundgren S, Ekse D, Varhaug JE, Mellgren G, Steen VM, Lien EA (2010) Associations between tamoxifen, estrogens, and FSH serum levels during steady state tamoxifen treatment of postmenopausal women with breast cancer. BMC Cancer 10:313
Borges S, Desta Z, Li L et al (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80:61–74
Lim JS, Chen XA, Singh O, Yap YS, Ng RC, Wong NS, Wong M, Lee EJ, Chowbay B (2011) Impact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patients. Br J Clin Pharmacol 71:737–750
Hosono N, Kato M, Kiyotani K et al (2009) CYP2D6 genotyping for functional-gene dosage analysis by allele copy number detection. Clin Chem 55:1546–1554
Rae JM, Drury S, Hayes DF et al. (2010) Lack of correlation between gene variants in tamoxifen metabolizing enzymes with primary endopoints in the ATAC trial. San Antonio Breast Cancer Symposium, San Antonio, Abstract S1–7
Leyland-Jones B, Regan MM, Bouzyk M et al. for the BIG 1-98 Collaborative and International Breast Cancer Study Groups. (2010) Outcome according to CYP2D6 genotype among postmenopausal women with endocrine-responsive early invasive breast cancer randomized in the BIG 1-98 trial. San Antonio Breast Cancer Symposium, San Antonio, Abstract S1–8
Irvin WJ, Jr., Walko CM, Weck KE et al. (2011) Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A Multicenter Study. J Clin Oncol 29(24):3232–3239
Day R (2001) Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. National surgical adjuvant breast and bowel project. Ann N Y Acad Sci 949:143–150
Henry NL, Rae JM, Li L et al (2009) Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat 117:571–575
Acknowledgments
This study was mainly supported by a Grant-in-Aid for Leading Project of Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was also supported in part by Grant-in-Aids for the Kobayashi Institute for Innovative Cancer Chemotherapy, the Japan Research Foundation for Clinical Pharmacology, the Takeda Science Foundation, and Young Scientists (B) (22790179) of Ministry of Education, Culture, Sports, Science and Technology of Japan. We express our heartfelt gratitude to all the study participants. We thank Yuka Kikuchi and Kumi Matsuda for technical assistance. We thank all other members and stuffs for their contribution to the sample collection and the completion of our study.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiyotani, K., Mushiroda, T., Imamura, C.K. et al. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat 131, 137–145 (2012). https://doi.org/10.1007/s10549-011-1777-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1777-7