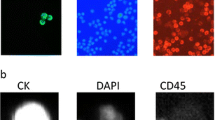
Abstract
Most assays to detect circulating tumor cells (CTCs) rely on EpCAM expression on tumor cells. Recently, our group reported that in contrast to other molecular breast cancer subtypes, “normal-like” cell lines lack EpCAM expression and are thus missed when CTCs are captured with EpCAM-based technology [J Natl Cancer Inst 101(1):61–66, 2009]. Here, the use of CD146 is introduced to detect EpCAM-negative CTCs, thereby improving CTC detection. CD146 and EpCAM expression were assessed in our panel of 41 breast cancer cell lines. Cells from 14 cell lines, 9 of which normal-like, were spiked into healthy donor blood. Using CellSearch™ technology, 7.5 ml whole blood was enriched for CTCs by adding ferrofluids loaded with antibodies against EpCAM and/or CD146 followed by staining for Cytokeratin and DAPI. Hematopoietic cells and circulating endothelial cells (CECs) were counterstained with CD45 and CD34, respectively. A similar approach was applied for blood samples of 20 advanced breast cancer patients. Eight of 9 normal-like breast cancer cell lines lacked EpCAM expression but did express CD146. Five of these 8 could be adequately recovered by anti-CD146 ferrofluids. Of 20 advanced breast cancer patients whose CTCs were enumerated with anti-EpCAM and anti-CD146 ferrofluids, 9 had CD146+ CTCs. Cells from breast cancer cell lines that lack EpCAM expression frequently express CD146 and can be recovered by anti-CD146 ferrofluids. CD146+ CTCs are present in the peripheral blood of breast cancer patients with advanced disease. Combined use of anti-CD146 and anti-EpCAM is likely to improve CTC detection in breast cancer patients.




Similar content being viewed by others
References
Sleijfer S, Gratama JW, Sieuwerts AM, Kraan J, Martens JW, Foekens JA (2007) Circulating tumour cell detection on its way to routine diagnostic implementation? Eur J Cancer 43(18):2645–2650
Mostert B, Sleijfer S, Foekens JA, Gratama JW (2009) Circulating tumor cells (CTCs): detection methods and their clinical relevance in breast cancer. Cancer Treat Rev 35(5):463–474
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7):1420–1430
HD SmerageJB (2008) The prognostic implications of circulating tumor cells in patients with breast cancer. Cancer Invest 26(2):109–114
Rack BK, Schindlbeck C, Schneeweiss A, Hilfrich J, Lorenz R, Beckmann MW, Pantel K, Lichtenegger W, Sommer HL, Janni WL (2008) Prognostic relevance of circulating tumor cells (CTCs) in peripheral blood of breast cancer patients before and after adjuvant chemotherapy: the German SUCCESS-Trial. J Clin Oncol 26 (May 20 suppl: abstr 503)
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JW, Gratama JW, Sleijfer S, Foekens JA (2009) Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 101(1):61–66
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114
Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Smid M, Timmermans M, Mostert B, Schutte M, Martens JW et al (2009) Response: Re: anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 101(12):896–897
Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, Maurer R, Metzger U, von Castelberg B, Bart R et al (2004) High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat 86(3):207–213
Zabouo G, Imbert AM, Jacquemier J, Finetti P, Moreau T, Esterni B, Birnbaum D, Bertucci F, Chabannon C (2009) CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res 11(1):1
Elstrodt F, Hollestelle A, Nagel JH, Gorin M, Wasielewski M, van den Ouweland A, Merajver SD, Ethier SP, Schutte M (2006) BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res 66(1):41–45
Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ et al (2009) Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat (Epub ahead of print)
Strijbos MH, Gratama JW, Kraan J, Lamers CH, den Bakker MA, Sleijfer S (2008) Circulating endothelial cells in oncology: pitfalls and promises. Br J Cancer 98(11):1731–1735
Brakenhoff RH, Stroomer JG, ten Brink C, de Bree R, Weima SM, Snow GB, van Dongen GA (1999) Sensitive detection of squamous cells in bone marrow and blood of head and neck cancer patients by E48 reverse transcriptase-polymerase chain reaction. Clin Cancer Res 5(4):725–732
Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75(12):2417–2426
Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M et al (2000) Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24(3):227–235
Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD (2007) MDA-MB-435 cells are derived from M14 melanoma cells–a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat 104(1):13–19
Chambers AF (2009) MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res 69(13):5292–5293
Rowand JL, Martin G, Doyle GV, Miller MC, Pierce MS, Connelly MC, Rao C, Terstappen LW (2007) Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A 71(2):105–113
Pickl WF, Majdic O, Fischer GF, Petzelbauer P, Fae I, Waclavicek M, Stockl J, Scheinecker C, Vidicki T, Aschauer H et al (1997) MUC18/MCAM (CD146), an activation antigen of human T lymphocytes. J Immunol 158(5):2107–2115
Smirnov DA, Foulk BW, Doyle GV, Connelly MC, Terstappen LW, O’Hara SM (2006) Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Res 66(6):2918–2922
SAGE anatomic viewer: KRT18 expression in vascular tissue. http://cgap.nci.nih.gov/SAGE/AnatomicViewer
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW et al (2006) Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409
Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A, Marty M (2008) Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 14(21):7004–7010
Bardin N, George F, Mutin M, Brisson C, Horschowski N, Frances V, Lesaule G, Sampol J (1996) S-Endo 1, a pan-endothelial monoclonal antibody recognizing a novel human endothelial antigen. Tissue antigens 48(5):531–539
Lehmann JM, Riethmuller G, Johnson JP (1989) MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA 86(24):9891–9895
Elshal MF, Khan SS, Raghavachari N, Takahashi Y, Barb J, Bailey JJ, Munson PJ, Solomon MA, Danner RL, McCoy JP Jr (2007) A unique population of effector memory lymphocytes identified by CD146 having a distinct immunophenotypic and genomic profile. BMC Immunol 8:29
Despoix N, Walzer T, Jouve N, Blot-Chabaud M, Bardin N, Paul P, Lyonnet L, Vivier E, Dignat-George F, Vely F (2008) Mouse CD146/MCAM is a marker of natural killer cell maturation. Eur J Immunol 38(10):2855–2864
Acknowledgments
The authors wish to thank Antoinette Hollestelle and Anita Trapman for their precious help in providing the immunohistochemistry data. This study was in part financially supported by the Netherlands Genomic Initiative (NGI)/Netherlands Organization for Scientific Research (NWO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mostert, B., Kraan, J., Bolt-de Vries, J. et al. Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res Treat 127, 33–41 (2011). https://doi.org/10.1007/s10549-010-0879-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0879-y