Abstract
Background
Despite bisphosphonate treatment, most patients with metastatic breast cancer will have either progressive bone metastases or skeletal related events (SREs). We evaluated the impact of second-line ibandronate on pain control and markers of bone turnover in these patients.
Methods
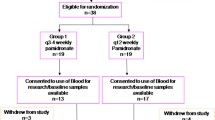
Patients with either an SRE or bony progression while on clodronate or intravenous (IV) pamidronate were switched to oral ibandronate 50 mg daily for 12 weeks. Pain scores and urinary N-telopeptide were evaluated weekly for 4 weeks and at weeks 8 and 12. There was no change in systemic anti-cancer treatment in the month before or after commencing study treatment. Palliative response was defined as a ≥ two-unit reduction in the worst pain score. Patient preferences between IV and oral bisphosphonate therapy were assessed.
Results
Thirty women completed the study. By week 12, patients experienced a significant improvement in pain control (OR = 0.41; P = 0.028) with 12 of 26 (46.2%) evaluable patients achieving a palliative response. Of the 23 patients who had received first-line IV pamidronate, 20 of 23 (87.0%) preferred oral therapy.
Conclusion
Patients with either progressive bone metastases or SREs while on clodronate or pamidronate may experience significant pain palliation with a switch to a more potent bisphosphonate. If confirmed by randomized trials, clinicians can start moving away from the paradigm whereby patients remain on a single bisphosphonate regimen throughout the course of their disease.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2006) Cancer statistics, 2005. CA Cancer J Clin 56(10):106–130
Plunkett TA, Smith P, Rubens RD (2000) Risk of complications from bone metastases in breast cancer: implications for management. Eur J Cancer 36:476–2
Ross JR, Saunders Y, Edmonds PM et al (2003) Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 327:469–475
Hillner B, Ingle JN, Chlebowski RT et al (2003) American Society of Clinical Oncology. American Society Of Clinical Oncology 2003 Update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 21:4042–4057
Jagdev SP, Purohit OP, Heatley S et al (2001) Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Ann Oncol 12: 1433–1438
Clemons M, Enright K, Cesta A et al (2004) Do physicians follow systemic treatment and funding policy guidelines? Can J Clin Pharmacol 11:168–178
Clemons M, Dranitsaris G, Ooi WS et al (2006) A phase II trial evaluating the palliative benefit of second-line Zoledronic acid in breast cancer patients with either a skeletal related event or progressive bone metastases despite first line bisphosphonate therapy. J Clin Oncol 24:4895–4900
Brown JE, Ellis SP, Gutcher SA et al (2005) Using bone turnover markers to direct bisphosphonate therapy: is this a feasible approach? Cancer Treat Rev 31:S30
Ali SM, Demers LM, Leitzel K et al (2004) Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastases. Ann Oncol 15:455–459
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23:4925–4935
Body JJ, Diel IJ, Lichinitzer M et al (2004) Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomized, placebo controlled phase III studies. Br J Cancer 90:1133–1137
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the brief pain inventory. Ann Acad Med 23:129–138
Cella DF, Tulsky DS, Gray G et al (1993) The Functional Assessment of Cancer Therapy (FACT) scale: development and validation of the general measure. J Clin Oncol 11:570–579
Repchinky C (ed) (2005) Compendium of pharmaceuticals and specialties. Canadian Pharmacist Association, Ottawa, ON
Allison PD (1999) Logistic regression using the sas system: theory and application; chapter 8. Cary, NC, SAS Institute Inc, p. 179–216
Major P, Cook R (2002) Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical end-points. Am J Clin Oncol 25:s10–s18
Gainford MC, Dranitsaris G, Clemons M (2005) Recent developments in bisphosphonates for patients with metastatic breast cancer. BMJ 330:769–773
Diel IJ (2005) Ibandronate: efficacy in the treatment of metastatic bone disease. Future Oncol 1:593–607
De Cock E, Hutton J, Canney P et al (2005) Cost-effectiveness of oral ibandronate versus IV zoledronic acid or IV pamidronate for bone metastases in patients receiving oral hormonal therapy for breast cancer in the United Kingdom. Clin Ther 27:1295–310
Bamias A, Kastritis E, Bamia E et al (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23:8580–8587
Clamp A, Danson S, Nguyen H et al (2004) Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol 5:607–16
Acknowledgments
We are grateful to Geetha Yogendran, Tatjana Sukovic, Betty YL Wong for their clinical and laboratory assistance with this project.
Initial findings from the study were presented as a late breaking poster presentation at The 29th Annual San Antonio Breast Cancer Symposium, December 8–11, 2006 in San Antonio, Texas. Abstract number: 3147.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clemons, M., Dranitsaris, G., Ooi, W. et al. A phase II trial evaluating the palliative benefit of second-line oral ibandronate in breast cancer patients with either a skeletal related event (SRE) or progressive bone metastases (BM) despite standard bisphosphonate (BP) therapy. Breast Cancer Res Treat 108, 79–85 (2008). https://doi.org/10.1007/s10549-007-9583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9583-y