Abstract
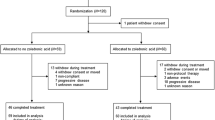
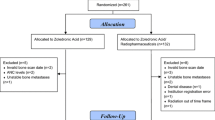
Previous studies suggest switching from pamidronate to a more potent bone-targeted agent is associated with biomarker and palliative response in breast cancer patients with bone metastases. Until now, this has not been addressed in a double-blind, randomized trial. Breast cancer patients with high-risk bone metastases, despite >3 months of pamidronate, were randomized to either continue pamidronate or switch to zoledronic acid every 4 weeks for 12 weeks. Primary outcome was the proportion of patients achieving a fall in serum C-telopeptide (sCTx) at 12 weeks. Secondary outcomes included difference in mean sCTx, pain scores, quality of life, toxicity, and skeletal-related events (SREs). Seventy-three patients entered the study; median age 61 years (range 37–87). Proportion of patients achieving a fall in sCTx over the 12-week evaluation period was 26/32 (81 %) with zoledronic acid and 18/29 (62 %) with pamidronate (p = 0.095). Mean decrease in sCTx (mean difference between groups = 50 ng/L, 95 % CI 18–84; p = 0.003) was significantly greater in patients who received zoledronic acid. Quality of life, pain scores, toxicity, and frequency of new SREs were comparable between the two arms. While a switch from pamidronate to zoledronic acid resulted in reduction in mean sCTx, there were no significant differences between the arms for proportion of patients achieving a reduction in sCTx, quality of life, pain scores, toxicity or SREs. Given the lack of palliative improvement, the current data do not support a switching strategy.



Similar content being viewed by others
References
Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD (1996) Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 335(24):1785–1791. doi:10.1056/NEJM199612123352401
Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M, Seaman JJ (2000) Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 88(5):1082–1090
Rosen LS, Gordon DH, Dugan W Jr, Major P, Eisenberg PD, Provencher L, Kaminski M, Simeone J, Seaman J, Chen BL, Coleman RE (2004) Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 100(1):36–43. doi:10.1002/cncr.11892
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman RE, Paterson AH, Gao GM, Kinsey AC, Peterson MC, Jun S (2008) Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res 14(20):6690–6696. doi:10.1158/1078-0432.CCR-07-5234
Wong MH, Stockler MR, Pavlakis N (2012) Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2:CD003474. doi:10.1002/14651858.CD003474.pub3
Clemons M, Gelmon KA, Pritchard KI, Paterson AH (2012) Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Curr Oncol (Tor, Ont) 19(5):259–268. doi:10.3747/co.19.1011
Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE, Theriault RL, Zuckerman DS, Von Roenn JH (2011) American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 29(9):1221–1227. doi:10.1200/jco.2010.32.5209
Verma S, Kerr-Cresswell D, Dranitsaris G, Charbonneau F, Trudeau M, Yogendran G, Cesta AM, Clemons M (2004) Bisphosphonate use for the management of breast cancer patients with bone metastases: a survey of Canadian Medical Oncologists. Support Care Cancer 12(12):852–858. doi:10.1007/s00520-004-0671-9
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma DJ, Chen BL, Seaman JJ (2003) Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 98(8):1735–1744. doi:10.1002/cncr.11701
Clemons MJ, Dranitsaris G, Ooi WS, Yogendran G, Sukovic T, Wong BY, Verma S, Pritchard KI, Trudeau M, Cole DE (2006) Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J Clin Oncol 24(30):4895–4900. doi:10.1200/JCO.2006.05.9212
Broom R, Du H, Clemons M, Eton D, Dranitsaris G, Simmons C, Ooi W, Cella D (2009) Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: measuring impact using the Functional Assessment of Cancer Therapy-Bone Pain. J Pain Symptom Manag 38(2):244–257. doi:10.1016/j.jpainsymman.2008.08.005
Clemons M, Dranitsaris G, Ooi W, Cole DE (2008) A phase II trial evaluating the palliative benefit of second-line oral ibandronate in breast cancer patients with either a skeletal related event (SRE) or progressive bone metastases (BM) despite standard bisphosphonate (BP) therapy. Breast Cancer Res Treat 108(1):79–85. doi:10.1007/s10549-007-9583-y
Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27(10):1564–1571. doi:10.1200/JCO.2008.19.2146
Amir E, Trinkaus M, Simmons CE, Dranitsaris G, Clemons MJ (2009) Vascular endothelial growth factor activity after switching of bisphosphonate treatment for metastatic breast cancer. J Clin Pathol 62(5):474–476. doi:10.1136/jcp.2008.062505
Amir E, Whyne C, Freedman OC, Fralick M, Kumar R, Hardisty M, Clemons M (2009) Radiological changes following second-line zoledronic acid treatment in breast cancer patients with bone metastases. Clin Exp Metastasis 26(5):479–484. doi:10.1007/s10585-009-9247-x
Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE (2003) Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer 89(11):2031–2037. doi:10.1038/sj.bjc.6601437
Coleman RE, Major P, Lipton A, Brown JE, Lee K-A, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23(22):4925–4935. doi:10.1200/jco.2005.06.091
Health Canada Vitamin D and Calcium: updated dietary reference intakes. http://www.hc-sc.gc.ca/fn-an/nutrition/vitamin/vita-d-eng.php#a7. Accessed 27 Oct 2014
Harris K, Li K, Flynn C, Chow E (2007) Worst, average or current pain in the Brief Pain Inventory: which should be used to calculate the response to palliative radiotherapy in patients with bone metastases? Clin Oncol 19(7):523–527. doi:10.1016/j.clon.2007.04.007
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Ford JA, Jones R, Elders A, Mulatero C, Royle P, Sharma P, Stewart F, Todd R, Mowatt G (2013) Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur J Cancer 49(2):416–430. doi:10.1016/j.ejca.2012.07.016
Brown JE, Cook RJ, Lipton A, Costa L, Coleman RE (2010) Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res Treat 123(3):767–779. doi:10.1007/s10549-010-0981-1
Coleman RE (2002) The clinical use of bone resorption markers in patients with malignant bone disease. Cancer 94(10):2521–2533
Jacobs C, Ng T, Ong M, Clemons M (2014) Long-term benefits versus side-effects from bone-targeted therapies for cancer patients: minimizing risk while maximizing benefits. Curr Opin Support Palliat Care 8(4):420–428. doi:10.1097/spc.0000000000000084
Vinholes JJ, Purohit OP, Abbey ME, Eastell R, Coleman RE (1997) Relationships between biochemical and symptomatic response in a double-blind randomised trial of pamidronate for metastatic bone disease. Ann Oncol 8(12):1243–1250
Acknowledgement
This study was funded by the Canadian Breast Cancer Foundation-Ontario Chapter, research project grant program.
Funding
The funding source had no role in study design, data collection, analysis, interpretation, or manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mark Clemons has received educational grants and travel sponsorship from Novartis. Susan Dent has received speaker honoraria from Novartis and attended advisory boards for Amgen. All other authors declare that they have no conflict of interest.
Additional information
Clinical Trials identifier: NCT01907880.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jacobs, C., Kuchuk, I., Bouganim, N. et al. A randomized, double-blind, phase II, exploratory trial evaluating the palliative benefit of either continuing pamidronate or switching to zoledronic acid in patients with high-risk bone metastases from breast cancer. Breast Cancer Res Treat 155, 77–84 (2016). https://doi.org/10.1007/s10549-015-3646-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3646-2