Abstract
Aromatase inhibitors are rapidly becoming the cornerstone of endocrine treatment for advanced disease and are now also used as adjuvant treatment in early-stage disease. The objective of this study was to assess the cost-effectiveness of adjuvant treatment with exemestane versus tamoxifen for early-stage breast cancer after 2–3 years treatment with tamoxifen in Sweden.
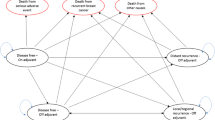
The results are based on findings in the Intergroup Exemestane Study (IES). IES was a randomized controlled trial in which postmenopausal women who had received 2–3 years of tamoxifen therapy following primary treatment of early-stage breast cancer, were randomized to either continue on tamoxifen therapy or be switched to exemestane therapy. The results showed a disease-free survival hazard ratio of exemestane relative to tamoxifen in IES of 0.69. A Markov state-transition model was developed to simulate consequences after the end of the clinical trial, and to integrate the trial data with external data on mortality, costs and quality of life specific for Swedish women.
The cost per QALY gained was about €20,000 in the base case analysis without inclusion of consequences of coronary heart disease. Inclusion of these events increased the cost-effectiveness ratio to about €31,000. This means that, based on our assumption, sequential exemestane treatment in early breast cancer is a cost-effective option compared with tamoxifen alone, although more long-term data on overall survival and consequences of adverse events would be valuable to increase the validity of the analysis further.
Similar content being viewed by others
References
Stewart B, Kleihues P (2003) World Health Organization. World Cancer Report. International Agency on Research for Cancer. IARC Press, Lyon, pp 188–190
National Board of Health and Welfare (2005) Cancer numbers, Stockholm
(1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351(9114):1451–1467
Wakeling AE, Valcaccia B, Newboult E et al (1984) Non-steroidal antioestrogens—receptor binding and biological response in rat uterus, rat mammary carcinoma and human breast cancer cells. J Steroid Biochem 20(1):111–120
Geisler J, Haynes B, Anker G et al (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20(3):751–757
Geisler J, King N, Anker G et al (1998) In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res 4(9):2089–2093
Bonneterre J, Buzdar A, Nabholtz JM et al (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92(9):2247–2258
Kaufmann M, Bajetta E, Dirix LY et al (2000) Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol 18(7):1399–1411
Mouridsen H, Gershanovich M, Sun Y et al (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21(11):2101–2109
Baum M, Budzar AU, Cuzick J et al (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359(9324):2131–2139
Clemons M, Coleman RE, Verma S (2004) Aromatase inhibitors in the adjuvant setting: bringing the gold to a standard? Cancer Treat Rev 30(4):325–332
Goss PE, Ingle JN, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J␣Med 349(19):1793–1802
Coombes RC, Hall E, Gibson LJ et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350(11):1081–1092
Carani C, Qin K, Simoni M et al (1997) Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337(2):91–95
Sirola J, Kroger H, Honkanen R et al (2003) Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas 45(3):159–167
Torgerson DJ, Bell-Syer SE (2001) Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA 285(22):2891–2897
Clemons M, Danson S, Howell A (2002) Tamoxifen (“Nolvadex”): a review. Cancer Treat Rev 28(4):165–180
Senkus-Konefka E, Konefka T, Jassem J (2004) The effects of tamoxifen on the female genital tract. Cancer Treat Rev 30(3):291–301
Costantino JP, Kuller LH, Ives DG et al (1997) Coronary heart disease mortality and adjuvant tamoxifen therapy. J␣Natl Cancer Inst 89(11):776–782
McDonald CC, Alexander FE, Whyte BW et al (1995) Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomised trial. The Scottish Cancer Trials Breast Group. Bmj 311(7011):977–980
McDonald CC, Stewart HJ (1991) Fatal myocardial infarction in the Scottish adjuvant tamoxifen trial. The Scottish Breast Cancer Committee. BMJ 303(6800):435–437
Nordenskjold B, Rosell J, Rutqvist LE et al (2005) Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: results from a randomized trial. J Natl Cancer Inst 97(21):1609–1610
Rutqvist LE, Mattsson A (1993) Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. The Stockholm Breast Cancer Study Group. J␣Natl Cancer Inst 85(17):1398–1406
(1996) Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst 88(21):1543–1549
(2005) Statistics Sweden. Sweden’s Statistical Databases
(2004) Karolinska University hospital, Stockholm, Sweden
(2005) Expert opinion, Dr Nils Wilking, Karolinska University Hospital
Läkemedelsinformation AB (2005) FASS Läkemedel i Sverige (Swedish drug prices). www. fass.se, Oslo
The Swedish Council on Technology Assessment in Health Care (SBU) (2003) Strålbehandling vid cancer. Rapport nr 162/2, Linköping
Lund University hospital (2004) Price list, Lund, Sweden
Remak E, Brazil L (2004) Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 91(1):77–83
Kanis JA, Johnell O, Oden A et al (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11(8):669–674
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312(7041):1254–1259
Singer BR, McLauchlan GJ, Robinson CM et al (1998) Epidemiology of fractures in 15,000 adults: the influence of age and gender. J Bone Joint Surg Br 80(2):243–248
Zethraeus N, Stromberg L, Jonsson B et al (1997) The cost of a hip fracture. Estimates for 1,709 patients in Sweden. Acta Orthop Scand 68(1):13–17
Centre for Health Economics (Stockholm School of Economics) (2002) Costs and quality of life associated with osteoporosis related fractures—Based on a Swedish pilot study. Stockholm
Holli K, Valavaara R, Blanco G et al (2000) Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer. Finnish Breast Cancer Group. J Clin Oncol 18(20):3487–3494
(2000) Vårdkostnader och vårdtider 2000 för NordDRG. Centrum för patient klassificering (CPK), Socialstyrelsen och Landstingsförbundet
Meltzer D (1997) Accounting for future costs in medical cost-effectiveness analysis. J Health Econ 16(1):33–64
Ekman M, Zethraeus N, Dahlstrom U et al (2002) [Cost-effectiveness of bisoprolol in chronic heart failure]. Lakartidningen 99(7):646–650
Lundberg L (1999) Health-related quality of life in Sweden. Dissertation, Faculty of Pharmacy, Uppsala University, Uppsala
Ashby J, O’Hanlon M, Buxton MJ (1994) The time trade-off technique: how do the valuations of breast cancer patients compare to those of other groups? Qual Life Res 3(4):257–265
Karnon J, Brown J (2002) Tamoxifen plus chemotherapy versus tamoxifen alone as adjuvant therapies for node- positive postmenopausal women with early breast cancer: a stochastic economic evaluation. Pharmacoeconomics 20(2):119–137
Karnon J, Johnson S, Delea T et al (2004) Cost-effectiveness of letrozole after five years of tamoxifen in postmenopausal women with early breast cancer. (Poster from ASCO June 2004)
Howell A, Cuzick J (2005) Vascular effects of aromatase inhibitors: data from clinical trials. J Steroid Biochem Mol Biol 95(1–5):143–149
(2003) Stockholms stads budgetavräkning, in Stadsledningskontorets redovisningsstab. www.stockholm.se
Centre for Health Economics (Stockholm School of Economics) (2004) A reassessment of the cost-effectiveness of hormone replacement therapy in Sweden—results based on the Women’s Health Initiative randomised controlled trial, Working paper 571, Stockholm
Cohen DJ, Taira DA, Berezin R et al (2001) Cost- effectiveness of coronary stenting in acute myocardial infarction: results from the stent primary angioplasty in myocardial infarction (stent-PAMI) trial. Circulation 104(25):3039–3045
Nicholson T, McGuire A, Milne R (2001) Cost-utility of enoxaparin compared with unfractionated heparin in unstable coronary artery disease. BMC Cardiovasc Disord 1(1):2
Zethraeus N, Johannesson M, Jonsson B (1999) A computer model to analyze the cost-effectiveness of hormone replacement therapy. Int J Technol Assess Health Care 15(2):352–365
(2004) Guide to the Methods of Technology Appraisal (reference N0515). National Institute for Clinical Excellence
Eichler HG, Kong SX, Gerth WC et al (2004) Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 7(5):518–528
Garber AM, Phelps CE (1997) Economic foundations of cost-effectiveness analysis. J Health Econ 16(1):1–31
Hillner BE (2004) Benefit and projected cost-effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer 101(6):1311–1322
(2005) Cancer Research UK (http://info.cancerresearchuk.org/)
Bundred NJ (2005) The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer 93(Suppl 1):S23–S27
Markopoulos C, Polychronis A, Zobolas V et al (2005) The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: preliminary results of the␣TEAM Greek sub-study. Breast Cancer Res Treat 93(1):61–66
Shilling V, Jenkins V, Fallowfield L et al (2003) The effects of hormone therapy on cognition in breast cancer. J Steroid Biochem Mol Biol 86(3–5):405–412
Karnon J, Delea T, Johnston SR et al (2006) Cost effectiveness of extended adjuvant letrozole in postmenopausal women after adjuvant tamoxifen therapy: the UK perspective. Pharmacoeconomics 24(3):237–250
Hussain SA, Williams S, Stevens A et al (2004) Endocrine therapy for early breast cancer. Expert Rev Anticancer Ther 4(5):877–888
Mouridsen HT, Robert NJ (2005) Benefit with aromatase inhibitors in the adjuvant setting for postmenopausal women with breast cancer. MedGenMed 7(3):20
Karnon J (2006) Aromatase inhibitors in breast cancer: a review of cost considerations and cost effectiveness. Pharmacoeconomics 24(3):215–232
Acknowledgements
Pfizer Sweden for financial support
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundkvist, J., Wilking, N., Holmberg, S. et al. Cost-effectiveness of exemestane versus tamoxifen as adjuvant therapy for early-stage breast cancer after 2–3 years treatment with tamoxifen in Sweden . Breast Cancer Res Treat 102, 289–299 (2007). https://doi.org/10.1007/s10549-006-9333-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9333-6