Abstract
Congenital disorders of glycosylation (CDG) are inborn errors of metabolism due to protein and lipid hypoglycosylation. This rapidly growing family of genetic diseases comprises 103 CDG types, with a broad phenotypic diversity ranging from mild to severe poly-organ -system dysfunction. This literature review summarizes cardiac involvement, reported in 20% of CDG. CDG with cardiac involvement were divided according to the associated type of glycosylation: N-glycosylation, O-glycosylation, dolichol synthesis, glycosylphosphatidylinositol (GPI)-anchor biosynthesis, COG complex, V-ATPase complex, and other glycosylation pathways. The aim of this review was to document and interpret the incidence of heart disease in CDG patients. Heart disorders were grouped into cardiomyopathies, structural defects, and arrhythmogenic disorders. This work may contribute to improved early management of cardiac complications in CDG.

Similar content being viewed by others
Abbreviations
- A:
-
Arrhythmia
- AD:
-
Aortic dilation
- AI:
-
Aorta insufficiency
- ASD:
-
Atrial septal defect
- ASH:
-
Atrial septal hypertrophy
- B:
-
Bradycardia
- BAV:
-
Bicuspid aortic valve
- BF:
-
Biventricular function
- BVD:
-
Biventricular dilation
- BVH:
-
Biventricular hypertrophy
- CD:
-
Cardiac dilation
- CDG:
-
Congenital disorder(s) of glycosylation
- CHD:
-
Congenital heart disease
- CM:
-
Cardiomegaly
- CVD:
-
Cardiac valve defects
- DCM:
-
Dilated cardiomyopathy
- ER:
-
Endoplasmic reticulum
- GPI:
-
Glycosylphosphatidylinositol
- HCM:
-
Hypertrophic cardiomyopathy
- HF:
-
Heart failure
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- LDCM:
-
Dilated ventricular cardiomyopathy
- LRHCM:
-
Left/right HCM
- LRVHCM:
-
Left/right ventricular HCM
- LVD:
-
Left ventricular dilation
- LVEF:
-
Left ventricular ejection fraction
- LVH:
-
Left ventricular hypertrophy
- LVRWMA:
-
Left ventricular regional wall motion abnormality (defined as a left ventricular segment in which the systolic motion score is below normal)
- MP:
-
Mitral prolapse
- MRI:
-
Magnetic resonance imaging
- PDA:
-
Patent ductus arteriosus
- PFO:
-
Patent foramen ovale
- PH:
-
Pulmonary hypertension
- PMV:
-
Parachute mitral valve
- PPS:
-
Peripheral pulmonary stenosis
- PVSD:
-
Perimembranous ventricular septal defect
- QRS:
-
Q wave, R wave, and S wave
- RAD:
-
Right atrial dilation
- RBBB:
-
Right bundle branch block
- RHHS:
-
Right hypoplastic heart syndrome
- RVD:
-
Right ventricular dilation
- RVDe:
-
Right ventricular defect
- RVEF:
-
Right ventricular ejection fraction
- RVH:
-
Right ventricular hypertrophy
- SAI:
-
Small aortic isthmus
- SD:
-
Septal defect
- SR:
-
Sarcoplasmic reticulum
- T:
-
Tachycardia
- TI:
-
Tricuspid insufficiency
- TR:
-
Tricuspid regurgitation
- VD:
-
Ventricular dysfunction
- VH:
-
Ventricular hypertrophy
- VLD:
-
Valvular defects
- VSD:
-
Ventricular septal defect
References
Alazami AM, Al-Qattan SM, Faqeih E (2016) Expanding the clinical and genetic heterogeneity of hereditary disorders of connective tissue. Hum Genet. doi:10.1007/s00439-016-1660-z
Al-Gazali L, Hertecant J, Algawi K, El Teraifi H, Dattani M (2008) A new autosomal recessive syndrome of ocular colobomas, ichthyosis, brain malformations and endocrine abnormalities in an inbred Emirati family. Am J Med Genet A 146A:813–819
Allali S, Le Goff C, Pressace-Diebold I et al (2011) Molecular screening of ADAMTSL2 gene in 33 patients reveals the genetic heterogeneity of geleophysic dysplasia. J Med Genet 48:417–421
Al-Owain M, Mohamed S, Kaya N, Zagal A, Matthijs G, Jaeken J (2010) A novel mutation and first report of dilated cardiomyopathy in ALG6-CDG (CDG-Ic): a case report. Orphanet J Rare Dis 5:7
AlSubhi S, AlHashem A, AlAzami A et al (2015) Further delineation of the ALG9-CDG phenotype. JIMD Rep 27:107–112
Althoff SS, Grueneberg M, Reunert J et al (2016) TMEM165 deficiency: postnatal changes in glycosylation. JIMD Rep 26:21–29
Arimura T, Inagaki N, Hayashi T et al (2009) Impaired binding of ZASP/cypher with phosphoglucomutase 1 is associated with dilated cardiomyopathy. Cardiovasc Res 83:80–88
Aronica E, van Kempen AA, van der Heide M et al (2005) Congenital disorder of glycosylation type Ia: a clinicopathological report of a newborn infant with cerebellar pathology. Acta Neuropathol 109:433–442
Ashraf GM, Bilal N, Suhail N, Hasan S, Banu N (2010) Glycosylation of purified buffalo heart galectin-1 plays crucial role in maintaining its structural and functional integrity. Biochemistry (Mosc) 75:1450–1457
Baasanjav S, Al-Gazali L, Hashiguchi T et al (2011) Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am J Hum Genet 89:15–27
Baycin-Hizal D, Gottschalk A, Jacobson E et al (2015) Physiologic and pathophysiologic consequences of altered sialylation and glycosylation on ion channel function. Biochem Biophys Res Commun 453:243–253
Bello L, Melacini P, Pezzani R et al (2012) Cardiomyopathy in patients with POMT1-related congenital and limb-girdle muscular dystrophy. Eur J Hum Genet 20:1234–1239
Boito CA, Melacini P, Vianello A et al (2005) Clinical and molecular characterization of patients with limb-girdle muscular dystrophy type 2I. Arch Neurol 62:1894–1899
Bourteel H, Vermersch P, Cuisset JM et al (2009) Clinical and mutational spectrum of limb-girdle muscular dystrophy type 2I in 11 French patients. J Neurol Neurosurg Psychiatry 80:1405–1408
Brady PD, Moerman P, De Catte L et al (2014) Exome sequencing identifies a recessive PIGN splice site mutation as a cause of syndromic congenital diaphragmatic hernia. Eur J Med Genet 57:487–493
Brockington M, Blake DJ, Prandini P et al (2001) Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet 69:1198–1209
Cantagrel V, Lefeber D (2011) From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J Inherit Metab Dis 34:859–867
Cantagrel V, Lefeber DJ, Ng BG et al (2010) SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell 142:203–217
Chang Y, Twiss JL, Horoupian DS, Caldwell SA, Johnston KM (1993) Inherited syndrome of infantile olivopontocerebellar atrophy, micronodular cirrhosis, and renal tubular microcysts: review of the literature and a report of an additional case. Acta Neuropathol 86:399–404
Clayton PT, Winchester BG, Keir G (1992) Hypertrophic obstructive cardiomyopathy in a neonate with the carbohydrate-deficient glycoprotein syndrome. J Inherit Metab Dis 15:857–861
Coman D, Bostock D, Hunter M, Kannu P, Irving M, Mayne V, Fietz M, Jaeken J, Savarirayan R (2008) Primary skeletal dysplasia as a major manifesting feature in an infant with congenital disorder of glycosylation type Ia. Am J Med Genet A 146A(3):389–392
Cotarelo RP, Valero MC, Prados B et al (2008) Two new patients bearing mutations in the fukutin gene confirm the relevance of this gene in Walker-Warburg syndrome. Clin Genet 73:139–145
D’Amico A, Petrini S, Parisi F et al (2008) Heart transplantation in a child with LGMD2I presenting as isolated dilated cardiomyopathy. Neuromuscul Disord 18:153–155
Dassie-Ajdid J, Causse A, Poidvin A et al (2009) Novel B3GALTL mutation in Peters-plus syndrome. Clin Genet 76:490–492
de Lonlay P, Seta N, Barrot S et al (2001) A broad spectrum of clinical presentations in congenital disorders of glycosylation I: a series of 26 cases. J Med Genet 38:14–19
Du D, Yang H, Ednie AR, Bennet ES (2017) In-silico modeling of the functional role of reduced sialylation in sodium and potassium channel gating of mouse ventricular myocytes. IEEE J Biomed Health Inform. doi:10.1109/JBHI.2017.2664579
Ednie AR, Bennett ES (2015) Reduced sialylation impacts ventricular repolarization by modulating specific K+ channel isoforms distinctly. J Biol Chem 290:2769–2783
Ednie AR, Horton KK, Wu J, Bennett ES (2013) Expression of the sialyltransferase, ST3Gal4, impacts cardiac voltage-gated sodium channel activity, refractory period and ventricular conduction. J Mol Cell Cardiol 59:117–127
Faletra F, Athanasakis E, Minen F et al (2011) Vertebral defects in patients with Peters plus syndrome and mutations in B3GALTL. Ophthalmic Genet 32:256–258
Fauth C, Steindl K, Toutain A et al (2016) A recurrent Germline mutation in the PIGA Gene causes Simpson–Golabi–Behmel syndrome type 2. Am J Med Genet A 170A:392–402
Feldman BJ, Rosenthal D (2002) Carbohydrate-deficient glycoprotein syndrome-associated pericardial effusion treated with corticosteroids and salicylic acid. Pediatr Cardiol 23:469–471
Fleming L, Lemmon M, Beck N et al (2016) Genotype-phenotype correlation of congenital anomalies in multiple congenital anomalies hypotonia seizures syndrome (MCAHS1)/PIGN-related epilepsy. Am J Med Genet A 170A:77–86
Foulquier F, Vasile E, Schollen E et al (2006) Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc Natl Acad Sci U S A 103:3764–3769
Funke S, Gardeitchik T, Kouwenberg D et al (2013) Perinatal and early infantile symptoms in congenital disorders of glycosylation. Am J Med Genet A 161A:578–584
Gehrmann J, Sohlbach K, Linnebank M et al (2003) Cardiomyopathy in congenital disorders of glycosylation. Cardiol Young 13:345–351
Haldeman-Englert CR, Naeem T, Geiger EA et al (2009) A 781-kb deletion of 13q12.3 in a patient with Peters plus syndrome. Am J Med Genet A 149A:1842–1845
Heinonen TYK, Pasternack L, Lindfors K et al (2003) A novel human glycosyltransferase: primary structure and characterization of the gene and transcripts. BBRC 309:166–174
Heinonen TYK, Pelto-Huikko M, Pasternack L et al (2006) Murine ortholog of the novel glycosyltransferase, B3GTL: primary structure, characterization of the gene and transcripts, and expression in tissues. DNA Cell Biol 8:465–474
Hollingsworth KG, Willis TA, Bates MG et al (2013) Subepicardial dysfunction leads to global left ventricular systolic impairment in patients with limb girdle muscular dystrophy 2I. Eur J Heart Fail 15:986–994
Işıkay S, Başpınar O, Yılmaz K (2014) A case of congenital disorder of glycosylation ia presented with recurrent pericardial effusion. Iran J Pediatr 24:652–654
Jaeken J, Morava E (2016) Congenital disorders of glycosylation and dolichol and glycosylphosphatidylinositol metabolism. In: Baumgartner W (ed) Inborn metabolic diseases. Diagnosis and treatment. Saudubray, 6th edn. Springer-Verlag, Berlin Heidelberg, pp 607–622
Job F, Mizumoto S, Smith L et al (2016) Functional validation of novel compound heterozygous variants in B3GAT3 resulting in severe osteopenia and fractures: expanding the disease phenotype. BMC Med Genet 17:86
Jones KL, Schwarze U, Adam MP, Byers PH, Mefford HC (2015) A homozygous B3GAT3 mutation causes a severe syndrome with multiple fractures, expanding the phenotype of linkeropathy syndromes. Am J Med Genet A 167A:2691–2696
Kapusta L, Zucker N, Frenckel G et al (2013) From discrete dilated cardiomyopathy to successful cardiac transplantation in congenital disorders of glycosylation due to dolichol kinase deficiency (DK1-CDG). Heart Fail Rev 18:187–196
Kasapkara CS, Tümer L, Ezgü FS et al (2012) SRD5A3-CDG: a patient with a novel mutation. Eur J Paediatr Neurol 16:554–556
Kefi M, Amouri R, Chabrak S, Mechmeche R, Hentati F et al (2008) Variable cardiac involvement in Tunisian siblings harboring FKRP gene mutations. Neuropediatrics 39:113–115
Kiarash A, Kelly CE, Phinney BS, Valdivia HH, Abrams J, Cala SE (2004) Defective glycosylation of calsequestrin in heart failure. Cardiovasc Res 63:264–272
Klcovansky J, Mørkrid L, Möller T (2016) Heart transplantation in a child with congenital disorder of glycosylation. J Heart Lung Transplant 35:1048–1049
Kranz C, Jungeblut C, Denecke J et al (2007a) A defect in dolichol phosphate biosynthesis causes a new inherited disorder with death in early infancy. Am J Hum Genet 80:433–440
Kranz C, Basinger AA, Güçsavas-Calikoglu M et al (2007b) Expanding spectrum of congenital disorder of glycosylation Ig (CDG-Ig): sibs with a unique skeletal dysplasia, hypogammaglobulinemia, cardiomyopathy, genital malformations, and early lethality. Am J Med Genet A 143A:1371–1378
Kristiansson B, Stibler H, Conradi N, Eriksson BO, Ryd W (1998) The heart and pericardial effusions in CDGS-I (carbohydrate-deficient glycoprotein syndrome type I). J Inherit Metab Dis 21:112–124
Kusa J, Pyrkosz A, Skiba A, Szkutnik M (2003) Cardiac manifestations of carbohydrate-deficient glycoprotein syndrome. Pediatr Cardiol 24:493–494
Kvarnung M, Nilsson D, Lindstrand A et al (2013) A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J Med Genet 50:521–528
Lam C, Golas GA, Davids M et al (2015) Expanding the clinical and molecular characteristics of PIGT-CDG, a disorder of glycosylphosphatidylinositol anchors. Mol Genet Metab 115:128–140
Le Goff C, Morice-Picard F, Dagoneau N et al (2008) ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-β bioavailability regulation. Nat Genet 40:1119–1123
Lefeber DJ, Schönberger J, Morava E et al (2009) Deficiency of Dol-P-man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am J Hum Genet 85:76–86
Lefeber DJ, de Brouwer AP, Morava E et al (2011) Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet 7:e1002427
Lesnik Oberstein SAJ, Kriek M, White SJ et al (2006) Peters plus Ssndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am J Hum Genet 79:562–566
Liang WC, Hayashi YK, Ogawa M et al (2013) Limb-girdle muscular dystrophy type 2I is not rare in Taiwan. Neuromuscul Disord 23:675–681
Lieu MT, Ng BG, Rush JS et al (2013) Severe, fatal multisystem manifestations in a patient with dolichol kinase-congenital disorder of glycosylation. Mol Genet Metab 110:484–489
Louhichi N, Triki C, Quijano-Roy S et al (2004) New FKRP mutations causing congenital muscular dystrophy associated with mental retardation and central nervous system abnormalities. Identification of a founder mutation in Tunisian families. Neurogenetics 5:27–34
Malhotra A, Pateman A, Chalmers R, Coman D, Menahem S (2009) Prenatal cardiac ultrasound finding in congenital disorder of glycosylation type 1a. Fetal Diagn Ther 25:54–57
Margeta M, Connolly AM, Winder TL, Pestronk A, Moore SA (2009) Cardiac pathology exceeds skeletal muscle pathology in two cases of limb-girdle muscular dystrophy type 2I. Muscle Nerve 40:883–889
Marquardt T, Hülskamp G, Gehrmann J, Debus V, Harms E, Kehl HG (2002) Severe transient myocardial ischemia caused by hypertrophic cardiomyopathy in a patient with congenital disorder of glycosylation type Ia. Eur J Pediatr 161:524–527
Marques-da-Silva D, Dos Reis FV, Monticelli M et al (2017) Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J Inherit Metab Dis. doi:10.1007/s10545-016-0012-4
Martinez HR, Craigen WJ, Ummat M, Adesina AM, Lotze TE, Jefferies JL (2014) Novel cardiovascular findings in association with a POMT2 mutation: three siblings with α-dystroglycanopathy. Eur J Hum Genet 22:486–491
Maydan G, Noyman I, Har-Zahav A et al (2011) Multiple congenital anomalies-hypotonia-seizures syndrome is caused by a mutation in PIGN. J Med Genet 48:383–389
McInerney-Leo AM, Harris JE, Gattas M et al (2016) Fryns syndrome associated with recessive mutations in PIGN in two separate families. Hum Mutat 37:695–702
Mercuri E, Brockington M, Straub V et al (2003) Phenotypic spectrum associated with mutations in the fukutin-related protein gene. Ann Neurol 53:537–542
Miura K, Shirasawa H (1987) Congenital muscular dystrophy of the Fukuyama type (FCMD) with severe myocardial fibrosis. A case report with postmortem angiography. Acta Pathol Jpn 37:1823–1835
Monticelli M, Ferro T, Jaeken J, Dos Reis FV, Videira PA (2016) Immunological aspects of congenital disorders of glycosylation (CDG): a review. J Inherit Metab Dis 39:765–780
Montpetit ML, Stocker PJ, Schwetz TA (2009) Regulated and aberrant glycosylation modulate cardiac electrical signaling. Proc Natl Acad Sci U S A 106:16517–16522
Morava E, Zeevaert R, Korsch E et al (2007) A common mutation in the COG7 gene with a consistent phenotype including microcephaly, adducted thumbs, growth retardation, VSD and episodes of hyperthermia. Eur J Hum Genet 15:638–645
Müller T, Krasnianski M, Witthaut R, Deschauer M, Zierz S (2005) Dilated cardiomyopathy may be an early sign of the C826A Fukutin-related protein mutation. Neuromuscul Disord 15:372–376
Munns CF, Fahiminiya S, Poudel N (2015) Homozygosity for frameshift mutations in XYLT2 result in a spondylo-ocular syndrome with bone fragility, cataracts, and hearing defects. Am J Hum Genet 96:971–978
Murakami T, Hayashi YK, Noguchi S et al (2006) Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann Neurol 60:597–602
Nagai-Okatani C, Minamino N (2016) Aberrant glycosylation in the left ventricle and plasma of rats with cardiac hypertrophy and heart failure. PLoS One 11:e0150210
Nakagawa Y, Nishikimi T, Kuwahara K et al (2017) MiR30-GALNT1/2 Axis-Mediated Glycosylation Contributes to the Increased Secretion of Inactive Human Prohormone for Brain Natriuretic Peptide (proBNP) From Failing Hearts. J Am Heart Assoc 6. doi:10.1161/JAHA.116.003601.
Nakanishi T, Sakauchi M, Kaneda Y et al (2006) Cardiac involvement in Fukuyama-type congenital muscular dystrophy. Pediatrics 117:e1187–e1192
Nakashima M, Kashii H, Murakami Y et al (2014) Novel compound heterozygous PIGT mutations caused multiple congenital anomalies-hypotonia-seizures syndrome 3. Neurogenetics 15:193–200
Noelle V, Knuepfer M, Pulzer F et al (2005) Unusual presentation of congenital disorder of glycosylation type 1a: congenital persistent thrombocytopenia, hypertrophic cardiomyopathy and hydrops-like aspect due to marked peripheral oedema. Eur J Pediatr 164:225–226
Pane M, Messina S, Vasco G et al (2012) Respiratory and cardiac function in congenital muscular dystrophies with alpha dystroglycan deficiency. Neuromuscul Disord 22:685–689
Petersen MB, Brostrøm K, Stibler H, Skovby F (1993) Early manifestations of the carbohydrate-deficient glycoprotein syndrome. J Pediatr 122(1):66–70
Poppe M, Bourke J, Eagle M (2004) Cardiac and respiratory failure in limb-girdle muscular dystrophy 2I. Ann Neurol 56:738–741
Reis LM, Tylor RC, Abdul-Rahman O et al (2008) Mutation analysis of B3GALTL in Peters plus syndrome. Am J Med Genet A 146A:2603–2610
Resende C, Carvalho C, Alegria A et al (2014) Congenital disorders of glycosylation with neonatal presentation. BMJ Case Rep
Rohlfing A-K, Rust S, Reunert J et al (2014) ALG1-CDG: a new case with early fatal outcome. Gene 534:345–351
Romano S, Bajolle F, Valayannopoulos V et al (2009) Conotruncal heart defects in three patients with congenital disorder of glycosylation type Ia (CDG Ia). J Med Genet 46:287–288
Rosales XQ, Moser SJ, Tran T (2011) Cardiovascular magnetic resonance of cardiomyopathy in limb girdle muscular dystrophy 2B and 2I. J Cardiovasc Magn Reson 13:39
Rudaks LI, Andersen C, Khong TY, Kelly A, Fietz M, Barnett CP (2012) Hypertrophic cardiomyopathy with cardiac rupture and tamponade caused by congenital disorder of glycosylation type Ia. Pediatr Cardiol 33:827–830
Rymen D, Peanne R, Millón MB et al (2013) MAN1B1 deficiency: an unexpected CDG-II. PLoS Genet 9:e1003989
Schollen E, Keldermans L, Foulquier F et al (2007) Characterization of two unusual truncating PMM2 mutations in two CDG-Ia patients. Mol Genet Metab 90:408–413
Schrapers E, Tegtmeyer LC, Simic-Schleicher G et al (2016) News on clinical details and treatment in PGM1-CDG. JIMD Rep 26:77–84
Shashi V, Zunich J, Kelly TE, Fryburg JS (1995) Neuroectodermal (CHIME) syndrome: an additional case with long term follow up of all reported cases. J Med Genet 32:465–469
Sparks SE, Krasnewich DM (2014) Congenital disorders of N-linked glycosylation. Pathway overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, LJH B, Bird TD, Fong CT, Mefford HC, RJH S, Stephens K (eds) GeneReviews® [Internet]. University of Washington, Seattle, pp 1993–2016
Strømme P, Maehlen J, Strøm EH, Torvik A (1991) Postmortem findings in two patients with the carbohydrate deficient glycoprotein syndrome. Acta Pediatr Scand Suppl 375:55–62
Sun L, Eklund EA, Chung WK et al (2005) Congenital disorder of glycosylation id presenting with hyperinsulinemic hypoglycemia and islet cell hyperplasia. J Clin Endocrinol Metab 90:4371–4375
Sveen ML, Schwartz M, Vissing J (2006) High prevalence and phenotype-genotype correlations of limb girdle muscular dystrophy type 2I in Denmark. Ann Neurol 59:808–815
Sveen ML, Thune JJ, Køber L, Vissing J (2008) Cardiac involvement in patients with limb-girdle muscular dystrophy type 2 and Becker muscular dystrophy. Arch Neurol 65:1196–1201
Tarailo-Graovac M, Sinclair G, Stockler-Ipsiroglu S et al (2015) The genotypic and phenotypic spectrum of PIGA deficiency. Orphanet J Rare Dis 10:23
Taylan F, Costantini A, Coles N et al (2016) Spondyloocular syndrome: novel mutations in XYLT2 Gene and Expansion of the phenotypic Spectrum. J Bone Miner Res 31:1577–1585
Tegtmeyer LC, Rust S, van Scherpenzeel M et al (2014) Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med 370:533–542
Tham E, Eklund EA, Hammarsjö A (2016) A novel phenotype in N-glycosylation disorders: Gillessen-Kaesbach-Nishimura skeletal dysplasia due to pathogenic variants in ALG9. Eur J Hum Genet 24:198–207
Thiel C, Körner C (2011) Mouse models for congenital disorders of glycosylation. J Inherit Metab Dis 34:879–889
Thong MK, Fietz M, Nicholls C, Lee MH, Asma O et al (2009) Congenital disorder of glycosylation type Ia in a Malaysian family: clinical outcome and description of a novel PMM2 mutation. J Inherit Metab Dis 32(Suppl 1):S41–S44
Timal S, Hoischen A, Lehle L et al (2012) Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Hum Mol Genet 21:4151–4161
Truin G, Guillard M, Lefeber DJ et al (2008) Pericardial and abdominal fluid accumulation in congenital disorder of glycosylation type Ia. Mol Genet Metab 94:481–484
Van Damme T, Gardeitchik T, Mohamed M et al (2017) Mutations in ATP6V1E1 or ATP6V1A cause autosomal-recessive cutis laxa. Am J Hum Genet 100:1–12
van de Kamp JM, Lefeber DJ, Ruijter GJ et al (2007) Congenital disorder of glycosylation type Ia presenting with hydrops fetalis. J Med Genet 44:277–280
Vasudevan D, Takeuchi H, Johar SS et al (2015) Peters plus syndrome mutations disrupt a noncanonical ER quality control mechanism. Curr Biol 25:286–295
Vesela K, Honzik T, Hansikova H (2009) A new case of ALG8 deficiency (CDG Ih). J Inherit Metab Dis 32 Suppl 1. doi:10.1007/s10545-009-1203-z.
Vleugels W, Keldermans L, Jaeken J et al (2009) Quality control of glycoproteins bearing truncated glycans in an ALG9-defective (CDG-IL) patient. Glycobiology 19:910–917
Vodovar N, Séronde MF, Laribi S et al (2014) Post-translational modifications enhance NT-proBNP and BNP production in acute decompensated heart failure. Eur Heart J 35:3434–3441
von Oettingen JE, Tan WH, Dauber A (2014) Skeletal dysplasia, global developmental delay, and multiple congenital anomalies in a 5-year-old boy-report of the second family with B3GAT3 mutation and expansion of the phenotype. Am J Med Genet A 164A:1580–1586
Wahbi K, Meune C, Hamouda el H et al (2008) Cardiac assessment of limb–girdle muscular dystrophy 2I patients: an echography, Holter ECG and magnetic resonance imaging study. Neuromuscul Disord 18:650–655
Walter MC, Petersen JA, Stucka R et al (2004) FKRP (826C>a) frequently causes limb-girdle muscular dystrophy in German patients. J Med Genet 41:e50
Weh E, Reis LM, Tyler RC et al (2014) Novel B3GALTL mutations in classic Peters plus syndrome and lack of mutations in a large cohort of patients with similar phenotypes. Clin Genet 86:142–148
Weinstein M, Schollen MG et al (2005) CDG-IL: an infant with a novel mutation in the ALG9 gene and additional phenotypic features. Am J Med Genet 136A:194–197
Wong SY, Beamer LJ, Gadomski T et al (2016) Defining the phenotype and assessing severity in phosphoglucomutase-1 deficiency. J Pediatr 175:130–136
Wu X, Steet RA, Bohorov O et al (2004) Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med 10:518–523
Yanagisawa A, Bouchet C, Van den Bergh PY et al (2007) New POMT2 mutations causing congenital muscular dystrophy: identification of a founder mutation. Neurology 69:1254–1260
Yilmaz A, Gdynia HJ, Ponfick M, Ludolph AC, Rösch S, Sechtem U (2011) The proteoglycan-dystrophin complex in genetic cardiomyopathies--lessons from three siblings with limb-girdle muscular dystrophy-2I (LGMD-2I). Clin Res Cardiol 100:611–615
Zeevaert R, Foulquier F, Cheillan D et al (2009) A new mutation in COG7 extends the spectrum of COG subunit deficiencies. Eur J Med Genet 52:303–305
Acknowledgments
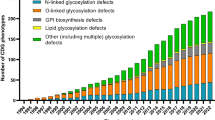
Dorinda Marques da Silva and Rita Francisco acknowledge support from the Liliana Scientific Scholarship 2016. We also thank the CDG & Allies—Professionals and Patient Associations International Network (CDG & Allies PPAIN), whose network expertise greatly helped with this manuscript. We are grateful to Diogo Sampaio (http://www.diogosampaio.pt/), who helped to design Fig. 1 of this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Vanessa dos Reis Ferreira is president and founder of the Portuguese Association for CDG and Other Rare Metabolic Diseases (APCDG-DMR). All other authors declare no competing financial interests.
Human and animal rights and informed consent
This review does not contain any data from human or animal studies performed by any of the listed authors.
Funding
This work was supported by the CDG Professionals and Patient Associations International Network (CDG & Allies—PPAIN) and Liliana Fellowships from APCDG attributed to Marques-da-Silva D. and Francisco R. Figure 1 was supported by Foundation Glycosylation. The authors confirmed independence from sponsors, the content of the article has not been influenced by sponsors. This work was supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-03687) grant to TP.
Additional information
Communicated by: Eva Morava
Note
This study is a result of a collaborative study between patient advocacy groups, families, and professionals (CDG Professionals and Patient Associations International Network; CDG & Allies—PPAIN).
Electronic supplementary material
ESM 1
(DOCX 73.6 kb)
Rights and permissions
About this article
Cite this article
Marques-da-Silva, D., Francisco, R., Webster, D. et al. Cardiac complications of congenital disorders of glycosylation (CDG): a systematic review of the literature. J Inherit Metab Dis 40, 657–672 (2017). https://doi.org/10.1007/s10545-017-0066-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0066-y