Abstract
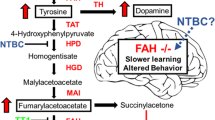
Tyrosinemia type I is a recessive inborn error of metabolism caused by mutations in the fumarylacetoacetate hydrolase (FAH) gene, coding for the final enzyme in the metabolism of tyrosine. This renders FAH nonfunctional and without treatment, toxic metabolites accumulate causing liver and kidney damage. Introduction of the drug NTBC in 2002 offered a treatment which inhibits an upstream enzyme, preventing the production of the toxic metabolites. There is now a long-term survival rate of greater than 90 % in children, but there are reports of lower cognitive function and IQ as well as schooling and behavioral problems in these children. We studied a mouse model of tyrosinemia type I to gain insight into the effects of tyrosinemia type I and treatment with NTBC on mouse learning, memory, and behavior. In the Barnes maze, visual and spatial cues can be used by mice to remember the location of a dark escape box. The primary time, distance, and strategy taken by the mice to locate the escape box is a measure of learning and memory. Our findings show that mice with tyrosinemia type I were slower to learn than wild-type mice treated with NTBC and made more mistakes, but were capable of learning and storing long-term memory. After learning the location of the target hole, mice with tyrosinemia type I respond differently to a change in location and were less flexible in learning the new target hole location. Our findings suggest that this slower learning and cognitive difference is caused by tyrosinemia type I and not by the treatment with NTBC.





Similar content being viewed by others
Abbreviations
- TT1:
-
Tyrosinemia type I
- FAH:
-
Fumarylacetoacetate hydrolase
- NTBC:
-
2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1-3-dione
- Tyr:
-
L-tyrosine
- Phe:
-
L-phenylalanine
- Trp:
-
L-tryptophan
- LNAA:
-
Large neutral amino acid
- HPPD:
-
4-hydroxyphenylpyruvate dioxygenase
- ALAD:
-
δ-aminolevulinate dehydratase
References
Adhikari A, Penatti CA, Resende RR et al (2006) 5-Aminolevulinate and 4, 5-dioxovalerate ions decrease GABA(A) receptor density in neuronal cells, synaptosomes and rat brain. Brain Res 1093(1):95–104
Aponte JL, Sega GA, Hauser LJ et al (2001) Point mutations in the murine fumarylacetoacetate hydrolase gene: Animal models for the human genetic disorder hereditary tyrosinemia type 1. Proc Natl Acad Sci U S A 98(2):641–645
Attar A, Liu T, Chan WT et al (2013) A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer's disease. PLoS One 8(11):e80355
Bach ME, Hawkins RD, Osman M et al (1995) Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell 81(6):905–915
Barnby E (2014) Tyrosinemia type 1: an overview of nursing care. Pediatr Nurs 40(2):61–68, 90
Barnes CA (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93(1):74–104
Bendadi F, de Koning TJ, Visser G et al (2014) Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J Pediatr 164(2):398–401
Brennan MJ, Cantrill RC (1979) Delta-aminolaevulinic acid is a potent agonist for GABA autoreceptors. Nature 280(5722):514–515
De Laet C, Munoz VT, Jaeken J et al (2011) Neuropsychological outcome of NTBC-treated patients with tyrosinaemia type 1. Dev Med Child Neurol 53(10):962–964
Deacon RM (2006) Assessing nest building in mice. Nat Protoc 1:1117–1119
Ellis MK, Whitfield AC, Gowans LA et al (1995) Inhibition of 4-hydroxyphenylpyruvate dioxygenase by 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione and 2-(2-chloro-4-methanesulfonylbenzoyl)-cyclohexane-1,3-dione. Toxicol Appl Pharmacol 133(1):12–19
Felitsyn N, McLeod C, Shroads AL et al (2008) The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J Neurochem 106(5):2068–2079
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370
Gibbs TC, Payan J, Brett AM et al (1993) Peripheral neuropathy as the presenting feature of tyrosinaemia type I and effectively treated with an inhibitor of 4-hydroxyphenylpyruvate dioxygenase. J Neurol Neurosurg Psychiatry 56(10):1129–1132
Halvorsen S, Pande H, Loken AC et al (1966) Tyrosinosis. A study of 6 cases. Arch Dis Child 41(217):238–249
Harding CO, Winn SR, Gibson KM et al (2014) Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU). J Inherit Metab Dis 37(5):735–743
Harrison FE, Reiserer RS, Tomarken AJ et al (2006) Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem 13(6):809–819
Jorquera R, Tanguay RM (2001) Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum Mol Genet 10(17):1741–1752
Kobayashi M, Nakamura T, Akai K (1980) A neuropathological investigation of a case of tyrosinosis. Acta Pathol Jpn 30(2):285–292
Kopin IJ, White JH, Bankiewicz K (1988) A new approach to biochemical evaluation of brain dopamine metabolism. Cell Mol Neurobiol 8(2):171–179
Labelle Y, Puymirat J, Tanguay RM (1993) Localization of cells in the rat brain expressing fumarylacetoacetate hydrolase, the deficient enzyme in hereditary tyrosinemia type 1. Biochim Biophys Acta 1180(3):250–256
Lambert GW, Eisenhofer G, Jennings GL et al (1993) Regional homovanillic acid production in humans. Life Sci 53(1):63–75
Lindblad B, Lindstedt S, Steen G (1977) On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A 74(10):4641–4645
Lindstedt S, Holme E, Lock EA et al (1992) Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340(8823):813–817
Lock EA, Gaskin P, Ellis MK et al (1996) Tissue distribution of 2-(2-nitro-4-trifluoromethylbenzoyl)cyclohexane-1-3-dione (NTBC): effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol Appl Pharmacol 141(2):439–447
Maiorana A, Malamisura M, Emma F et al (2014) Early effect of NTBC on renal tubular dysfunction in hereditary tyrosinemia type 1. Mol Genet Metab 113(3):188–193
Masurel-Paulet A, Poggi-Bach J, Rolland MO et al (2008) NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis 31(1):81–87
Mitchell G, Larochelle J, Lambert M et al (1990) Neurologic crises in hereditary tyrosinemia. N Engl J Med 322(7):432–437
Müller WE, Snyder SH (1977) delta-Aminolevulinic acid: influences on synaptic GABA receptor binding may explain CNS symptoms of porphyria. Ann Neurol 2(4):340–342
Nave KA (2010) Myelination and support of axonal integrity by glia. Nature 468(7321):244–252
Neve S, Aarenstrup L, Tornehave D et al (2003) Tissue distribution, intracellular localization and proteolytic processing of rat 4-hydroxyphenylpyruvate dioxygenase. Cell Biol Int 27(8):611–624
Noble-Jamieson G, Jamieson N, Clayton P et al (1994) Neurological crisis in hereditary tyrosinaemia and complete reversal after liver transplantation. Arch Dis Child 70(6):544–545
O'Leary TP, Brown RE (2013) Optimization of apparatus design and behavioral measures for the assessment of visuo-spatial learning and memory of mice on the Barnes maze. Learn Mem 20(2):85–96
Partington MW, Haust MD (1967) A patient with tyrosinemia and hypermethioninemia. Can Med Assoc J 97(18):1059–1067
Pickar D, Breier A, Kelsoe J (1988) Plasma homovanillic acid as an index of central dopaminergic activity: studies in schizophrenic patients. Ann N Y Acad Sci 537:339–346
Pohorecka M, Biernacka M, Jakubowska-Winecka A et al (2012) Behavioral and intellectual functioning in patients with tyrosinemia type I. Pediatr Endocrinol Diabetes Metab 18(3):96–100
Santra S, Preece MA, Hulton SA et al (2008) Renal tubular function in children with tyrosinaemia type I treated with nitisinone. J Inherit Metab Dis 31(3):399–402
Sassa S, Kappas A (1983) Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest 71(3):625–634
Thimm E, Herebian D, Assmann B et al (2011) Increase of CSF tyrosine and impaired serotonin turnover in tyrosinemia type I. Mol Genet Metab 102(2):122–125
Thimm E, Richter-Werkle R, Kamp G et al (2012) Neurocognitive outcome in patients with hypertyrosinemia type I after long-term treatment with NTBC. J Inherit Metab Dis 35(2):263–268
Van Spronsen FJ, Thomasse Y, Smit GP et al (1994) Hereditary tyrosinemia type I: a new clinical classification with difference in prognosis on dietary treatment. Hepatology 20(5):1187–1191
Wyss PA, Boynton S, Chu J et al (1993) Tissue distribution of succinylacetone in the rat in vivo: a possible basis for neurotoxicity in hereditary infantile tyrosinemia. Biochim Biophys Acta 1182(3):323–328
Acknowledgments
The authors are extremely grateful to Melanie Klarer, Wolfgang Langhans and Urs Meyer (Physiology and Behavior Laboratory, ETH Zurich, Switzerland) for teaching us the art of mouse behavioral experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Megan Hillgartner, Sarah Coker, Ashton Koenig, Marissa Moore, Elizabeth Barnby and Gordon MacGregor declare that they have no conflict of interest.
Animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed. The care, housing, breeding, and maze experiments of animals were approved by the UAH IACUC committee.
Funding
This study was funded internally by UAH.
Additional information
Communicated by: Nenad Blau
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 494 kb)
Rights and permissions
About this article
Cite this article
Hillgartner, M.A., Coker, S.B., Koenig, A.E. et al. Tyrosinemia type I and not treatment with NTBC causes slower learning and altered behavior in mice. J Inherit Metab Dis 39, 673–682 (2016). https://doi.org/10.1007/s10545-016-9949-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-016-9949-6