Abstract
Congenital disorders of glycosylation (CDG) are a rapidly growing family of genetic diseases caused by defects in glycosylation. Nearly 100 CDG types are known so far. Patients present a great phenotypic diversity ranging from poly- to mono-organ/system involvement and from very mild to extremely severe presentation. In this literature review, we summarize the liver involvement reported in CDG patients. Although liver involvement is present in only a minority of the reported CDG types (22 %), it can be debilitating or even life-threatening. Sixteen of the patients we collated here developed cirrhosis, 10 had liver failure. We distinguish two main groups: on the one hand, the CDG types with predominant or isolated liver involvement including MPI-CDG, TMEM199-CDG, CCDC115-CDG, and ATP6AP1-CDG, and on the other hand, the CDG types associated with liver disease but not as a striking, unique or predominant feature, including PMM2-CDG, ALG1-CDG, ALG3-CDG, ALG6-CDG, ALG8-CDG, ALG9-CDG, PGM1-CDG, and COG-CDG. This review aims to facilitate CDG patient identification and to understand CDG liver involvement, hopefully leading to earlier diagnosis, and better management and treatment.

Similar content being viewed by others
Abbreviations
- AF:
-
Alpha-fetoprotein
- AP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AT:
-
Antithrombin
- CDG:
-
Congenital disorder(s) of glycosylation
- CK:
-
Creatine kinase
- ER:
-
Endoplasmic reticulum
- ERGIC:
-
Endoplasmic reticulum-Golgi intermediate compartment
- γ-GT:
-
Gamma-glutamyl transferase
- IEF:
-
Isoelectrofocusing
- PT:
-
Prothrombin time
- PTT:
-
Partial thromboplastin time
- TA:
-
Transaminases
References
AlSubhi S, AlHashem A, AlAzami A et al (2015) Further delineation of the ALG9-CDG phenotype. JIMD Rep 27:107–112
Arnoux JB, Boddaert N, Valayannopoulos V et al (2008) Risk assessment of acute vascular events in congenital disorder of glycosylation type Ia. Mol Genet Metab 93:444–449
Aronica E, van Kempen AAMW, van der Heide M et al (2005) Congenital disorder of glycosylation type Ia: a clinicopathological report of a newborn infant with cerebellar pathology. Acta Neuropathol 109:433–442
Babovic-Vuksanovic D, Patterson MC, Schwenk WF et al (1999) Severe hypoglycemia as a presenting symptom of carbohydrate-deficient glycoprotein syndrome. J Pediatr 135:775–781
Barone R, Sturiale L, Fiumara A, Uziel G, Garozzo D, Jaeken J (2007) Borderline mental development in a congenital disorder of glycosylation (CDG) type Ia patient with multisystemic involvement (intermediate phenotype). J Inherit Metab Dis 30:107
Blomme B, Van Steenkiste C, Callewaert N, Van Vlierberghe H (2009) Alteration of protein glycosylation in liver diseases. J Hepatol 50:592–603
Chantret I, Dancourt J, Dupre T et al (2003) A deficiency in dolichyl-P-glucose: Glc1Man9GlcNAc2-PP-dolichyl alpha3-glucosyltransferase defines a new subtype of congenital disorders of glycosylation. J Biol Chem 298:9962–9971
Choi R, Woo HI, Choe B-H (2015) Application of whole exome sequencing to a rare inherited metabolic disease with neurological and gastrointestinal manifestations: a congenital disorder of glycosylation mimicking glycogen storage disease. Clin Chim Acta 444:50–53
Damen G, de Klerk H, Huijmans J, den Hollander J, Sinaasappel M (2004) Gastrointestinal and other clinical manifestations in 17 children with congenital disorders of glycosylation type Ia, Ib, and Ic. J Pediatr Gastroenterol Nutr 38:282–287
de Koning TJ, Dorland L, van Diggelen OP et al (1998) A novel disorder of N-glycosylation due to phosphomannose isomerase deficiency. Biochem Biophys Res Commun 245:38–42
de Lonlay P, Seta N (2009) The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim Biophys Acta 1792:841–843
de Lonlay P, Seta N, Barrot S et al (2001) A broad spectrum of clinical presentations in congenital disorders of glycosylation I: a series of 26 cases. J Med Genet 38:14–19
Eklund EA, Sun L, Westphal V, Northrop JL, Freeze HH, Scaglia F (2005) Congenital disorder of glycosylation (CDG)-Ih patient with a severe hepato-intestinal phenotype and evolving central nervous system pathology. J Pediatr 147:847–850
Eklund EA, Sun L, Yang SP, Pasion RM, Thorland EC, Freeze HH (2006) Congenital disorder of glycosylation Ic due to a de novo deletion and an hALG-6 mutation. Biochem Biophys Res Commun 339:755–760
Ferro JM, Viana P, Santos P (2016) Management of neurologic manifestations in patients with liver disease. Curr Treat Options Neurol 18:37
Foulquier F, Vasile E, Schollen E et al (2006) Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. PNAS 103:3764–3769
Frank CG, Grubenmann CE, Eyaid W, Berger EG, Aebi M, Hennet T (2004) Identification and functional analysis of a defect in the human ALG9 Gene: definition of congenital disorder of glycosylation type IL. Am J Hum Genet 75:146–150
Freeze HH, Chong JX, Bamshad MJ, Ng BG (2014) Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet 94:161–175
Fung CW, Matthijs G, Sturiale L et al (2012) COG5-CDG with a mild neurohepatic presentation. JIMD Rep 3:67–70
Grubenmann CE, Frank CG, Hülsmeier AJ et al (2004) Deficiency of the first mannosylation step in the N-glycosylation pathway causes congenital disorder of glycosylation type Ik. Hum Mol Genet 13:535–542
Grünewald S (2009) The clinical spectrum of phosphomannomutase 2 deficiency (CDG-Ia). Biochim Biophys Acta 1792:827–834
Grünewald S, De Vos R, Jaeken J (2003) Abnormal lysosomal inclusions in liver hepatocytes but not in fibroblasts in congenital disorders of glycosylation (CDG). J Inherit Metab Dis 26:49–54
Hendriksz CJ, McClean P, Henderson MJ et al (2001) Successful treatment of carbohydrate deficient glycoprotein syndrome type 1b with oral mannose. Arch Dis Child 85:339–340
Hernández EM, Vega Pajares AIV, González BP et al (2008) Defecto congénito de glucosilación tipo Ib. experiencia en el tratamiento con manosa. An Pediatr (Barc) 69:358–365
Höck M, Wegleiter K, Ralser E et al (2015) ALG8-CDG: novel patients and review of the literature. Orphanet J Rare Dis 10:73–80
Jaeken J, Morava E (2016) Congenital disorders of glycosylation and dolichol and glycosylphosphatidylinositol metabolism. In: Saudubray, Baumgartner, Walter (ed) Inborn metabolic diseases. diagnosis and treatment, 6th edn. Springer, Berlin, pp 607–622
Jaeken J, Stibler H, Hagberg B (1991) The carbohydrate-deficient glycoprotein syndrome. a new inherited multisystemic disease with severe nervous system involvement. Acta Paediatr Scand Suppl 375:1–71
Jaeken J, Matthijs G, Saudubray J-M et al (1998) Phosphomannose isomerase deficiency: a carbohydrate-deficient glycoprotein syndrome with hepatic-intestinal presentation. Am J Hum Genet 62:1535–1539
Jaeken J, Lefeber D, Matthijs G (2014) Clinical utility gene card for: phosphomannose isomerase deficiency. Eur J Hum Genet. doi:10.1038/ejhg.2014.29
Jaeken J, Lefeber D, Matthijs G (2015a) Clinical utility gene card for: ALG1 defective congenital disorder of glycosylation. Eur J Hum Genet. doi:10.1038/ejhg.2015.9
Jaeken J, Lefeber D, Matthijs G (2015b) Clinical utility gene card for: ALG6 defective congenital disorder of glycosylation. Eur J Hum Genet. doi:10.1038/ejhg.2014.146
Jansen JC, Timal S, van Scherpenzeel M et al (2016a) TMEM199 deficiency is a disorder of Golgi homeostasis characterized by elevated aminotransferases, alkaline phosphatase, and cholesterol and abnormal glycosylation. Am J Hum Genet 98:322–330
Jansen JC, Cirak S, van Scherpenzeel M et al (2016b) CCDC115 deficiency causes a disorder of golgi homeostasis with abnormal protein glycosylation. Am J Hum Genet 98:310–321
Jansen EJR, Timal S, Ryan M et al (2016c) ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation. Nat Commun 7:11600
Janssen MCH, de Kleine RH, van den Berg AP et al (2014) Successful liver transplantation and long-term follow-up in a patient with MPI-CDG. Pediatrics 134:e279–e283
Kelly DF, Boneh A, Pitsch S et al (2001) Carbohydrate-deficient glycoprotein syndrome 1b: a new answer to an old diagnostic dilemma. J Paediatr Child Health 37:510–512
Kjaergaard S, Schwartz M, Skovby F (2001) Congenital disorder of glycosylation type Ia (CDG-Ia): phenotypic spectrum of the R141H/F119L genotype. Arch Dis Child 85:236–239
Kodera H, Ando N, Yuasa I et al (2015) Mutations in COG2 encoding a subunit of the conserved oligomeric golgi complex cause a congenital disorder of glycosylation. Clin Genet 87:455–460
Kornak U, Reynders E, Dimopoulou A et al (2008) Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet 40:32–34
Lepais L, Cheillan D, Frachon SC et al (2015) ALG3-CDG: report of two siblings with antenatal features carrying homozygous p.Gly96Arg Mutation. Am J Med Genet 167A:2748–2754
Liem YS, Bode L, Freeze HH, Leebeek FWG, Zandbergen AAM, Wilson JHP (2008) Using heparin therapy to reverse protein-losing enteropathy in a patient with CDG-Ib. Nat Clin Pract Gastroenterol Hepatol 5:220–224
Mention K, Lacaille F, Valayannopoulos V et al (2008) Development of liver disease despite mannose treatment in two patients with CDG-Ib. Mol Genet Metabol 93:40–43
Miura Y, Tay SKH, Aw MM, Eklund E, Freeze HH (2005) Clinical and biochemical characterization of a patient with congenital disorder of glycosylation (CDG) IIX. J Pediatr 147:851–853
Monin ML, Mignot C, De Lonlay P et al (2014) 29 French adult patients with PMM2-congenital disorder of glycosylation: outcome of the classical pediatric phenotype and depiction of a late-onset phenotype. Orphanet J Rare Dis 9:207
Monticelli M, Ferro T, Jaeken J, Dos Reis Ferreira V, Videira PA (2016) Immunological aspects of congenital disorders of glycosylation (CDG): a review. J Inherit Metab Dis 39:765–780
Morava E (2014) Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol Genet Metab 112:275–279
Morava E, Zeevaert R, Korsch E et al (2007) A common mutation in the COG7 gene with a consistent phenotype including microcephaly, adducted thumbs, growth retardation, VSD and episodes of hyperthermia. Eur J Hum Genet 15:638–665
Morava E, Vodopiutz J, Lefeber DJ et al (2012) Defining the phenotype in congenital disorder of glycosylation due to ALG1 mutations. Pediatrics 130:e1034–e1039
Morava E, Tiemes V, Thiel C et al (2016) ALG6-CDG: a recognizable phenotype with epilepsy, proximal muscle weakness, ataxia and behavior and limb anomalies. J Inherit Metab Dis 39:713–723
Ng BG, Kranz C, Hagebeuk EEO et al (2007) Molecular and clinical characterization of a Moroccan Cog7 deficient patient. Mol Genet Metab 91:201–204
Ng BG, Sharma V, Sun L et al (2011) Identification of the first COG-CDG patient of Indian origin. Mol Genet Metab 102:364–367
Ng BG, Shiryaev SA, Rymen D et al (2016) ALG1-CDG: clinical and molecular characterization of 39 unreported patients. Hum Mutat 37:653–660
Niehues R, Hasilik M, Alton G et al (1998) Carbohydrate-deficient glycoprotein syndrome type Ib. phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 101:1414–1420
Ono H, Sakura N, Yamashita K, Yuasa I, Ohno K (2003) Novel nonsense mutation (R194X) in the PMM2 gene in a Japanese patient with congenital disorder of glycosylation type Ia. Brain Dev 27:525–528
Panneerselvam K, Freeze HH (1996) Mannose enters mammalian cells using a specific transporter that is insensitive to glucose. J Biol Chem 271:9417–9421
Reynders E, Foulquier F, Leão Teles E et al (2009) Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum Mol Genet 18:3244–3256
Rohlfing A-K, Rust S, Reunert J et al (2014) ALG1-CDG: a new case with early fatal outcome. Gene 534:345–351
Rymen D, Winter J, Van Hasselt PM et al (2015) Key features and clinical variability of COG6-CDG. Mol Genet Metabol 116:163–170
Schollen E, Frank CG, Keldermans L et al (2004) Clinical and molecular features of three patients with congenital disorders of glycosylation type Ih (CDG-Ih) (ALG8 deficiency). J Med Genet 41:550–556
Serrano M, de Diego V, Muchart J et al (2015) Phosphomannomutase deficiency (PMM2-CDG): ataxia and cerebellar assessment. Orphanet J Rare Dis 10:138
Shanti B, Silink M, Bhattacharya K et al (2009) Congenital disorder of glycosylation type Ia: heterogeneity in the clinical presentation from multivisceral failure to hyperinsulinaemic hypoglycaemia as leading symptoms in three infants with phosphomannomutase deficiency. J Inherit Metab Dis 32:S241–S251
Sorte H, Mørkrid L, Rødningen O et al (2012) Severe ALG8-CDG (CDG-Ih) associated with homozygosity for two novel missense mutations detected by exome sequencing of candidate genes. Eur J Med Genet 55:196–202
Spaapen LJM, Bakker JA, Van Der Meer SB et al (2005) Clinical and biochemical presentation of siblings with COG-7 deficiency, a lethal multiple O- and N-glycosylation disorder. J Inherit Metab Dis 28:707–714
Sparks SE, Krasnewich DM (2014) Congenital disorders of N-linked glycosylation. pathway overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K (eds) eneReviews®. University of Washington, Seattle, pp 1993–2016
Sun L, Eklund EA, Chung WK, Wang C, Cohen J, Freeze HH (2005) Congenital disorder of glycosylation Id presenting with hyperinsulinemic hypoglycemia and islet cell hyperplasia. J Clin Endocrinol Metab 90:4371–4375
Tegtmeyer LC, Rust S, van Scherpenzeel M et al (2014) Multiple phenotypes in phosphoglucomutase 1 deficiency. N Eng J Med 370:533–542
Vesela K, Honzik T, Hansikova H et al (2009) A new case of ALG8 deficiency (CDG Ih). J Inherit Metab Dis 32:259–264
Vleugels W, Keldermans L, Jaeken J et al (2009) Quality control of glycoproteins bearing truncated glycans in an ALG9-defective (CDG-IL) patient. Glycobiology 19:910–917
Weinstein M, Schollen, Matthijs G et al (2005) CDG-IL: an infant with a novel mutation in the ALG9 gene and additional phenotypic features. Am J Med Genet 136A:194–197
Westphal V, Kjaergaard S, Davis JÁ, Peterson SM, Skovby F, Freeze HH (2001) Genetic and metabolic analysis of the first adult with congenital disorder of glycosylation type Ib: long-term outcome and effects of mannose supplementation. Mol Genet Metabol 73:77–85
Wong SY, Beamer LJ, Gadomski T et al (2016) Defining the phenotype and assessing severity in phosphoglucomutase-1 deficiency. J Pediatr 175:130–136
Wu X, Steet RA, Bohorov O et al (2004) Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med 10:518–523
Zeevaert R, Foulquier F, Cheillan D et al (2009) A new mutation in COG7 extends the spectrum of COG subunit deficiencies. Eur J Med Genet 52:303–305
Acknowledgements
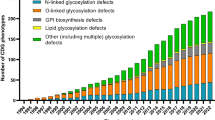
Dorinda Marques da Silva acknowledges support from the “Second Liliana Scientific Scholarship 2016”. We also thank the CDG & Allies – Professionals and Patient Associations International Network (CDG & Allies PPAIN), whose network expertise greatly helped this manuscript. We are grateful to Diogo Sampaio (http://www.diogosampaio.pt/), who helped design Fig. 1 of this publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
Vanessa dos Reis Ferreira is President and founder of the Portuguese Association for CDG and other Rare Metabolic Diseases (APCDG-DMR). All other authors declare no competing financial interests.
Funding
This work was supported by the CDG Professionals and Patient Associations International Network(CDG & Allies – PPAIN) and Liliana Fellowships from APCDG attributed to Marques-da-Silva D. and Monticelli M. The authors confirmed independence from the sponsors, the content of the article has not been influenced by sponsors.
Additional information
Communicated by: Eva Morava
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 69 kb)
Rights and permissions
About this article
Cite this article
Marques-da-Silva, D., dos Reis Ferreira, V., Monticelli, M. et al. Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J Inherit Metab Dis 40, 195–207 (2017). https://doi.org/10.1007/s10545-016-0012-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-016-0012-4