Abstract
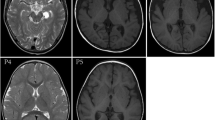
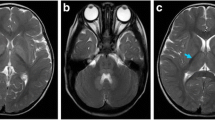
Valine, leucine, and isoleucine are essential branched chain amino acids (BCAAs). When BCAA metabolism is genetically impaired in human, serum levels of BCAA and/or their metabolites rise considerably, causing severe neurological dysfunction. The first step in BCAA catabolism is catalyzed by branched chain aminotransferase (BCAT). Hypervalinemia and hyperleucine-isoleucinemia caused by BCAT gene mutation in human have not been reported previously. A 25-year-old man presented with headache complaints and mild memory impairment for about six years. Brain MRI showed symmetric white matter abnormal signals. Metabolic studies revealed remarkably elevated plasma valine and leucine concentrations. Maple syrup urine disease (MSUD) diagnosis was not supported since all genes for the branched-chain α-keto acid dehydrogenase complex (BCKD) gene were normal. Interestingly, two heterogeneous BCAT2 gene mutations were found in the patient, including c.509G > A (p.Arg170Gln) and c.790G > A (p.Glu264Lys). In addition, c.509G > A (p.Arg170Gln) and c.790G > A (p.Glu264Lys) were found in his father and mother, respectively, suggesting an autosomal recessive disorder. BCAT2 functional studies demonstrated that the two BCAT2 gene mutations resulted in decreased BCAT2 enzyme activity. After treatment with vitamin B6, the levels of BCAA, especially valine were remarkably decreased and brain MRI lesions were improved. These findings suggest a new type of branched chain amino acid metabolism disorder. This rare case provides great insight into the further understanding of BCAA metabolism and its defect in human. BCAT2 gene mutations can cause hypervalinemia and hyperleucine-isoleucinemia, which are associated with brain white matter lesions.



Similar content being viewed by others
References
Barschak AG, Sitta A, Deon M et al (2009) Amino acids levels and lipid peroxidation in maple syrup urine disease patients. Clin Biochem 42:462–466
Brosnan JT, Brosnan ME (2006) Branched-chain amino acids: enzyme and substrate regulation. J Nutr 136:207S–211S
Chace DH, Hillman SL, Millington DS, Kahler SG, Roe CR, Naylor EW (1995) Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem 41:62–68
Conway ME, Yennawar N, Wallin R, Poole LB, Hutson SM (2002) Identification of a peroxide-sensitive redox switch at the CXXC motif in the human mitochondrial branched chain aminotransferase. Biochemistry 41:9070–9078
Conway ME, Yennawar N, Wallin R, Poole LB, Hutson SM (2003) Human mitochondrial branched chain aminotransferase: structural basis for substrate specificity and role of redox active cysteines. Biochim Biophys Acta 1647:61–65
Davoodi J, Drown PM, Bledsoe RK, Wallin R, Reinhart GD, Hutson SM (1998) Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases. J Biol Chem 273:4982–4989
Funchal C, Latini A, Jacques-Silva MC et al (2006) Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Neurochem Int 49:640–650
Hull J, Hindy ME, Kehoe PG, Chalmers K, Love S, Conway ME (2012) Distribution of the branched chain aminotransferase proteins in the human brain and their role in glutamate regulation. J Neurochem 123:997–1009
Hutson SM, Sweatt AJ, Lanoue KF (2005) Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr 135:1557S–1564S
Jan W, Zimmerman RA, Wang ZJ, Berry GT, Kaplan PB, Kaye EM (2003) MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology 45:393–399
Lu J, Xie G, Jia W (2013) Insulin resistance and the metabolism of branched-chain amino acids. Front Med 7:53–59
Schonberger S, Schweiger B, Schwahn B, Schwarz M, Wendel U (2004) Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab 82:69–75
Sgaravatti AM, Rosa RB, Schuck PF et al (2003) Inhibition of brain energy metabolism by the alpha-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta 1639:232–238
Strauss KA, Morton DH (2003) Branched-chain ketoacyl dehydrogenase deficiency: maple syrup disease. Curr Treat Options Neurol 5:329–341
Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM (2004) Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab 286:E64–E76
Tom A, Nair KS (2006) Assessment of branched-chain amino acid status and potential for biomarkers. J Nutr 136:324S–330S
Wada Y (1965) Idiopathic hypervalinemia: valine and alpha keto-acids in blood following an oral dose of valine. Tohoku J Exp Med 87:322–331
Wada Y, Tada K, Minagawa A, Yoshida T, Morikawa T, Okamura T (1963) Idiopathic hypervalinemia: probably a new entity of inborn error of valine metabolism. Tohoku J Exp Med 81:46–55
Wu JY, Kao HJ, Li SC et al (2004) ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest 113:434–440
Yudkoff M (1997) Brain metabolism of branched-chain amino acids. Glia 21:92–98
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (20131107120002). We acknowledge Professor Yanling Yang and Peng Yu for providing metabolite analyses.
Compliance with Ethics Guidelines
ᅟ
Conflict of interest
None.
Human and animal rights and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (the Institutional Review Board of Xuan Wu Hospital, Capital Medical University, Beijing, China) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Written informed consent was obtained from the patient.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jerry Vockley
Rights and permissions
About this article
Cite this article
Wang, X.L., Li, C.J., Xing, Y. et al. Hypervalinemia and hyperleucine-isoleucinemia caused by mutations in the branched-chain-amino-acid aminotransferase gene. J Inherit Metab Dis 38, 855–861 (2015). https://doi.org/10.1007/s10545-015-9814-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-015-9814-z