Summary
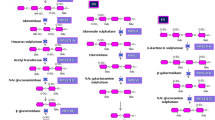
Glycosaminoglycans are accumulated in both mucopolysaccharidoses (MPS) and mucolipidoses (ML). MPS I, II, III and VII and ML II and ML III patients cannot properly degrade heparan sulphate (HS). In spite of the importance of HS storage in the metabolic pathway in these diseases, blood and urine HS levels have not been determined systematically using a simple and economical method. Using a new ELISA method using anti-HS antibodies, HS concentrations in blood and urine were determined in MPS and ML II and ML III patients. HS concentrations were determined in 156 plasma samples from MPS I (n = 23), MPS II (n = 26), MPS III (n = 24), MPS IV (n = 62), MPS VI (n = 5), MPS VII (n = 5), ML II (n = 8) and ML III (n = 3), and 205 urine samples from MPS I (n = 33), MPS II (n = 33), MPS III (n = 30), MPS IV (n = 82), MPS VI (n = 7), MPS VII (n = 9), ML II (n = 8) and ML III (n = 3). The ELISA method used monoclonal antibodies against HS. MPS I, II, III and VII and ML II and III patients had significant elevation in plasma HS, compared to the age-matched controls (p < 0.0001). Eighty-three out of 89 (93.3%) of individual values in the above MPS types and ML were above the mean +2SD of the controls. In urine samples, 75% of individual values in patients with those types were above the mean +2SD of the controls. In contrast to the previous understanding of the HS metabolic pathway, plasma HS levels in all five MPS VI and 15% of MPS IV patients were elevated above the mean +2SD of the controls. These findings suggest that HS concentration determined by ELISA, especially in plasma, could be a helpful marker for detection of the most severe MPS I, II, III, VI and VII and ML II, distinguishing them from normal populations.
Similar content being viewed by others
References
Byers S, Rozaklis T, Brumfield LK, Ranieri E, Hopwood JJ (1998) Glycosaminoglycan accumulation and excretion in the mucopolysaccharidoses: characterization and basis of a diagnostic test for MPS. Mol Genet Metab 65: 282–290.
Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S, Hopwood JJ (1997) Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J Clin Invest 99: 651–662.
David G (1993) Integral membrane heparan sulfate proteoglycans. FASEB J 7: 1023–1030.
Fuller M, Meikle PJ, Hopwood JJ (2004) Glycosaminoglycan degradation fragments in mucopolysaccharidosis I. Glycobiology 14: 443–450.
Harmatz P, Whitley CB, Waber L, et al (2004) Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux–Lamy syndrome). J Pediatr 144: 574–580.
Kakkis ED, Muenzer J, Tiller GE, et al (2001) Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med 344: 182–188.
Kornfeld S, Sly WS (2001) I-cell disease and pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc. eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 3469–3482.
Leteux C, Chai W, Nagai K, et al (2001) 10E4 antigen of scrapie lesions contains an unusual nonsulfated heparan motif. J Biol Chem 276: 12539–12545.
Lindahl U, Kusche-Gullberg M, Kjellen L (1998) Regulated diversity of heparan sulfate. J Biol Chem 273: 24979–24982.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF (1999) Prevalence of lysosomal storage disorders. JAMA 281: 249–254.
Meikle PJ, Ranieri ER, Simonsen H, et al (2004) Newborn screening for lysosomal storage disorders: clinical evaluation of a two-tier strategy. Pediatrics 114(4): 909–916.
Muenzer J, Fisher A (2004) Advances in the treatment of mucopolysaccharidosis type I. N Engl J Med 350: 1932–1934.
Muenzer J, Lamsa JC, Garcia A, et al (2002) Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr Suppl 439: 98–99.
Neufeld EF, Muenzer J (2001) The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc. eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 3421–3452.
Oguma T, Toyoda H, Toida T, Imanari T (2001) Analytical method of chondroitin/dermatan sulfates using high performance liquid chromatography/turbo ionspray ionization mass spectrometry: application to analyses of the tumor tissue sections on glass slides. Biomed Chromatogr 15: 356–362.
Ramsay SL, Meikle PJ, Hopwood JJ (2003) Determination of monosaccharides and disaccharides in mucopolysaccharidoses patients by electrospray ionisation mass spectrometry. Mol Genet Metab 78: 193–204.
Tomatsu S, Okamura K, Taketani T, et al (2004) Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res 55: 592–597.
Van den Born J, Gunnarsson K, Bakker MAH, et al (1995) Presence of N-unsubstituted glucosamine units in native heparan sulfate revealed by a monoclonal antibody. J Biol Chem 270: 31303–31309.
Wiesmann UN, Spycher MA, Meier C, Liebaers I, Herschkowitz N (1980) Prenatal mucopolysaccharidosis II (Hunter): a pathogenetic study. Pediatr Res 14: 749–756.
Wraith EJ, Clarke LA, Beck M, et al (2004) Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double blinded, placebo-controlled, multinational study of recombinant human α-l-iduronidase (laronidase). J Pediatr 144: 581–588.
Wraith EJ, Hopwood JJ, Fuller M, et al (2005) Laronidase treatment of mucopolysaccharidosis I. BioDrugs 19: 1–7.
Yanagishita M, Hascall VC (1992) Cell surface heparan sulfate proteoglycans. J Biol Chem 267: 9451–9454.
Yoshida K, Miyauchi S, Kikuchi H, Tawada A, Tokuyasu K (1989) Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid chromatography. Anal Biochem 177: 327–332.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomatsu, S., Gutierrez, M.A., Ishimaru, T. et al. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. J Inherit Metab Dis 28, 743–757 (2005). https://doi.org/10.1007/s10545-005-0069-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10545-005-0069-y