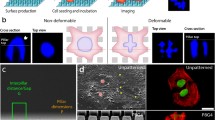
Abstract
Various micro-devices have been used to assess single cell mechanical properties. Here, we designed and implemented a novel, mechanically actuated, two dimensional cell culture system that enables a measure of cell stiffness based on quantitative functional imaging of cell-substrate interaction. Based on parametric finite element design analysis, we fabricated a soft (5 kPa) polydimethylsiloxane (PDMS) cell substrate coated with collagen-I and fluorescent micro-beads, thus providing a favorable terrain for cell adhesion and for substrate deformation quantification, respectively. We employed a real-time tracking system that analyzes high magnification images of living cells under stretch, and compensates for gross substrate motions by dynamic adjustment of the microscope stage. Digital image correlation (DIC) was used to quantify substrate deformation beneath and surrounding the cell, leading to an estimate of cell stiffness based upon the ability of the cell to resist the applied substrate deformation. Sensitivity of the system was tested using chemical treatments to both “soften” and “stiffen” the cell cytoskeleton with either 0.5 μg/ml Cytochalasin-D or 3% Glutaraldehyde, respectively. Results indicate that untreated osteosarcoma cells (SAOS-2) exhibit a 1.5 ± 0.7% difference in strain from an applied target substrate strain of 8%. Compared to untreated cells, those treated with Cyochalasin-D passively followed the substrate (0.5 ± 0.5%, p < 0.001), whereas Glutaraldehyde enhanced cellular stiffness and the ability to resist the substrate deformation (2.9 ± 1.6%, p < 0.001). Nano-indentation testing showed differences in cell stiffness based on culture treatment, consistent with DIC findings. Our results indicate that mechanics and image analysis approaches do hold promise as a method to quantitatively assess tensile cell constitutive properties.









Similar content being viewed by others
References
I. Arganda-Carreras, C.O.S. Sorzano et al., Consistent and elastic registration of histological sections using vector-spline regularization. Comput Vis Approaches Med Image Anal 4241, 85–95 (2006)
K.A. Barbee, E.J. Macarak et al., Strain measurements in cultured vascular smooth muscle cells subjected to mechanical deformation. Ann Biomed Eng 22(1), 14–22 (1994)
F.H. Bieler, C.E. Ott et al., Biaxial cell stimulation: A mechanical validation. J Biomech 42(11), 1692–1696 (2009)
S.Y. Chou, C.M. Cheng et al., Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials 30(18), 3136–3142 (2009)
S.E. Cross, Y.S. Jin et al., Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol 2(12), 780–783 (2007)
S.E. Cross, Y.S. Jin, et al. AFM-based analysis of human metastatic cancer cells. Nanotechnology. 19(38), (2008)
E.M. Darling, S. Zauscher et al., A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: Do cell properties reflect metastatic potential? Biophys J 92(5), 1784–1791 (2007)
M. Dembo, Y.L. Wang, Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76(4), 2307–2316 (1999)
E.K. Dimitriadis, F. Horkay et al., Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J 82(5), 2798–2810 (2002)
D. Docheva, D. Padula et al., Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J Cell Mol Med 12(2), 537–552 (2008)
A.J. Foulkes, R.C. Miall, Adaptation to visual feedback delays in a human manual tracking task. Exp Brain Res 131(1), 101–110 (2000)
A. Gerstmair, G. Fois et al., A device for simultaneous live cell imaging during uni-axial mechanical strain or compression. J Appl Physiol 107(2), 613–620 (2009)
B.W. Graf, S.A. Boppart, Imaging and analysis of three-dimensional cell culture models. Methods Mol Biol 591, 211–227 (2010)
J. Guck, S. Schinkinger et al., Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88(5), 3689–3698 (2005)
J.H. Hoh, C.A. Schoenenberger, Surface morphology and mechanical properties of MDCK monolayers by atomic force microscopy. J Cell Sci 107(Pt 5), 1105–1114 (1994)
R. Krishnan, C.Y. Park et al., Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE 4(5), e5486 (2009)
G.Y. Lee, C.T. Lim, Biomechanics approaches to studying human diseases. Trends Biotechnol 25(3), 111–118 (2007)
C.Y. Li, S.Y. Gao et al., In vitro assays for adhesion and migration of osteoblastic cells (Saos-2) on titanium surfaces. Cell Tissue Res 324(3), 369–375 (2006)
C.T. Lim, E.H. Zhou et al., Mechanical models for living cells—A review. J Biomech 39(2), 195–216 (2006)
B. Lincoln, H.M. Erickson et al., Deformability-based flow cytometry. Cytom A 59(2), 203–209 (2004)
B. Lincoln, S. Schinkinger et al., Reconfigurable microfluidic integration of a dual-beam laser trap with biomedical applications. Biomed Microdevices 9(5), 703–710 (2007)
Y. Mao, J.E. Schwarzbauer, Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci 118(Pt 19), 4427–4436 (2005)
T. Masuda, I. Takahashi et al., Development of a cell culture system loading cyclic mechanical strain to chondrogenic cells. J Biotechnol 133(2), 231–238 (2008)
U. Mayr-Wohlfart, J. Fiedler et al., Proliferation and differentiation rates of a human osteoblast-like cell line (SaOS-2) in contact with different bone substitute materials. J Biomed Mater Res 57(1), 132–139 (2001)
S. Munevar, Y.L. Wang et al., Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys J 80(4), 1744–1757 (2001)
T. Ochalek, F.J. Nordt et al., Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res 48(18), 5124–5128 (1988)
A.A. Patel, R.G. Thakar et al., Biophysical mechanisms of single-cell interactions with microtopographical cues. Biomed Microdevices 12(2), 287–296 (2010)
M. Paulitschke, G.B. Nash, Membrane rigidity of red-blood-cells parasitized by different strains of plasmodium-falciparum. J Lab Clin Med 122(5), 581–589 (1993)
D.B. Serrell, T.L. Oreskovic et al., A uniaxial bioMEMS device for quantitative force-displacement measurements. Biomed Microdevices 9(2), 267–275 (2007)
D. Shin, K. Athanasiou, Cytoindentation for obtaining cell biomechanical properties. J Orthop Res 17(6), 880–890 (1999)
P.G. Smith, R. Garcia et al., Strain reorganizes focal adhesions and cytoskeleton in cultured airway smooth muscle cells. Exp Cell Res 232(1), 127–136 (1997)
J.G. Snedeker, G. Pelled et al., Endoscopic cellular microscopy for in vivo biomechanical assessment of tendon function. J Biomed Opt 11(6), 064010 (2006)
D. Stamenovic ‡, Rheological behavior of mammalian cells. Cell Mol Life Sci 65(22), 3592–3605 (2008)
S. Suresh, Biomechanics and biophysics of cancer cells. Acta Biomater 3(4), 413–438 (2007)
S. Suresh, J. Spatz et al., Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater 1(1), 15–30 (2005)
J.L. Tan, J. Tien et al., Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA 100(4), 1484–1489 (2003)
W. Tan, T.A. Desai, Layer-by-layer microfluidics for biomimetic three-dimensional structures. Biomaterials 25(7–8), 1355–1364 (2004)
J.P. Vande Geest, E.S. Di Martino et al., An analysis of the complete strain field within Flexercell membranes. J Biomech 37(12), 1923–1928 (2004)
B. Wacogne, C. Pieralli et al., Measuring the mechanical behaviour of human oocytes with a very simple SU-8 micro-tool. Biomed Microdevices 10(3), 411–419 (2008)
M.E. Wall, P.S. Weinhold et al., Comparison of cellular strain with applied substrate strain in vitro. J Biomech 40(1), 173–181 (2007)
Y.L. Wang, R.J. Pelham Jr., Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol 298, 489–496 (1998)
P.J. Wipff, H. Majd et al., The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials 30(9), 1781–1789 (2009)
W.J. Yao, L. Gu et al., Wild type p53 gene causes reorganization of cytoskeleton and, therefore, the impaired deformability and difficult migration of murine erythroleukemia cells. Cell Motil Cytoskeleton 56(1), 1–12 (2003)
C. Zhu, G. Bao et al., Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng 2, 189–226 (2000)
Acknowledgments
The authors gratefully acknowledge Professor Janos Vörös for use of the AFM. We also thank Mr. Hansruedolf Sommer for his contribution to device construction, and Pascal Bissig and Michael Bieri from the ETH Zurich for their contribution to the tracking system design and implementation.
This study was funded by the Swiss National Science Foundation, grant number 205321-118036.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartalena, G., Grieder, R., Sharma, R.I. et al. A novel method for assessing adherent single-cell stiffness in tension: design and testing of a substrate-based live cell functional imaging device. Biomed Microdevices 13, 291–301 (2011). https://doi.org/10.1007/s10544-010-9493-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-010-9493-3