Abstract
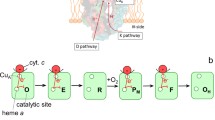
It is a pleasure to contribute to the special issue published in honor of Vladimir Skulachev, a distinguished scientist who greatly contributes to maintain a high standard of biochemical research in Russia. A more particular reason can be found in his work (Artzabanov, V. Y., Konstantinov, A. A., and Skulachev, V. P. (1978) FEBS Lett., 87, 180–185), where observations anticipating some ideas presented in my article were reported. Cytochrome c oxidase exhibits protonmotive, redox linked allosteric cooperativity. Experimental observations on soluble bovine cytochrome c oxidase are presented showing that oxido-reduction of heme a/CuA and heme a 3/CuB is linked to deprotonation/protonation of two clusters of protolytic groups, A1 and A2, respectively. This cooperative linkage (redox Bohr effect) results in the translocation of 1 H+/oxidase molecule upon oxido-reduction of heme a/CuA and heme a 3/CuB, respectively. Results on liposome-reconstituted oxidase show that upon oxidation of heme a/CuA and heme a 3/CuB protons from A1 and A2 are released in the outer aqueous phase. A1 but not A2 appears to take up protons from the inner aqueous space upon reduction of the respective redox center. A cooperative model is presented in which the A1 and A2 clusters, operating in close sequence, constitute together the gate of the proton pump in cytochrome c oxidase.
Similar content being viewed by others
REFERENCES
Monod, J., Wyman, J., and Changeux, I. P. (1965) J. Mol. Biol., 12, 88–118.
Perutz, M. F. (1976) Br. Med. Bull., 32, 193–194.
Perutz, M. F., Kilmartin, J. V., Nishikura, K., Fogg, J. H., Butler, P. J., and Rollema, H. S. (1980) J. Mol. Biol., 138, 649–668.
Kilmartin, J. V., and Rossi Bernardi, L. (1973) Physiol. Rev., 53, 836–890.
Dutton, P. L., and Wilson, D. F. (1974) Biochim. Biophys. Acta, 346, 165–212.
Clark, W. M. (1960) Oxidation-Reduction Potentials of Organic Systems, William and Wilkins Co, Baltimore, USA.
Papa, S. (1976) Biochim. Biophys. Acta, 456, 39–84.
Papa, S., Guerrieri, F., Lorusso, M., Izzo, G., Boffoli, D., and Maida, I. (1981) in Vectorial Reactions in Electron and Ion Transport (Palmieri, F., et al., eds.) Elsevier/North Holland Biomedical Press, p. 57.
Rousseau, D., Sassaroli, M., Ching, Y., and Dasgupta, S. (1988) Ann. N. Y. Acad. Sci., 550, 223–237.
Brzezinski, P., and Larsson, G. (2003) Biochim. Biophys. Acta, 1605, 1–13.
Brzezinski, P. (2004) Trends Biochem. Sci., 29, 380–387.
Tsukihara, T., Shimokata, K., Katayama, Y., Shimada, H., Muramoto, K., Aoyama, H., Mochizuki, M., Shinzawa-Itoh, K., Yamashita, E., Yao, M., Ishimura, Y., and Yoshikawa, S. (2003) PNAS, 100, 15304–15309.
Papa, S. (1989) Highlights in Modern Biochemistry (Kotyk, A., et al., eds.), Vol. 1, pp. 781–796.
Heberle, J. (2000) Biochim. Biophys. Acta, 1458, 135–147.
Wikstrom, M., Krab, K., and Saraste, M. (1981) Cytochrome c Oxidase. A Synthesis, Academic Press, London, pp. 111–115.
Van Gelder, B. F., van Rijin, J. L. M. L., Schilder, G. J. A., and Wilms, J. (1977) in Structure and Function of Energy-Transduction Membranes (van Dam, K., and van Gelder, B. F., eds.) Elsevier/North Holland, Amsterdam, pp. 61–68.
Ellis, W. R., Wang, H., Blair, D. F., Gray, H. B., and Chan, S. I. (1986) Biochemistry, 25, 161–167.
Nicholls, P., and Petersen, L. C. (1974) Biochim. Biophys. Acta, 357, 462–467.
Yoshikawa, S., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., Yamashita, E., Inoue, N., Yao, M., Fei, M., Libeu, C. P., Mizushima, T., Yamaguchi, H., Tomizaki, T., and Tsukihara, T. (1998) Science, 280, 1723–1729.
Kanti Das, T., and Mazundar, S. (2000) Biopolymers (Biospectroscopy), 57, 316–322.
Capitanio, N., Piccoli, C., Capitanio, G., Perna, G., Boffoli, D., Capozzi, V., and Papa, S. (2005) Physica Scripta, in press.
Callahan, P. M., and Babcock, G. T. (1983) Biochemistry, 22, 452–461.
Capitanio, N., Capitanio, G., Minuto, M., De Nitto, E., Palese, L. L., Nicholls, P., and Papa, S. (2000) Biochemistry, 39, 6373–6379.
Forte, E., Barone, M. C., Brunori, M., Sarti, P., and Giuffre, A. (2002) Biochemistry, 41, 13046–13052.
Forte, E., Scandurra, F. M., Richter, O. M. H., D’Itri, E., Sarti, P., Brunori, M., Ludvig, B., and Giuffre, A. (2004) Biochemistry, 43, 2957–2963.
Louro, R. O., Catarino, T., Le Gall, J., Turner, D. L., and Xavier, A. V. (2001) Chem. Biochem., 2, 22–28.
Papa, S., Capitanio, N., and Capitanio, G. (2004) Biochim. Biophys. Acta, 1655, 353–364.
Xavier, A. V. (2004) Biochim. Biophys. Acta, 1658, 23–30.
Capitanio, N., Capitanio, G., Boffoli, D., and Papa, S. (2000) Biochemistry, 39, 15454–15461.
Capitanio, N., Capitanio, G., Demarinis, D. A., De Nitto, E., Massari, S., and Papa, S. (1996) Biochemistry, 35, 10800–10806.
Papa, S., Capitanio, N., Capitanio, G., and Palese, L. L. (2004) Biochim. Biophys. Acta, 1658, 95–105.
Artzabanov, V. Y., Konstantinov, A. A., and Skulachev, V. P. (1978) FEBS Lett., 87, 180–185.
Capitanio, N., Capitanio, G., De Nitto, E., Boffoli, D., and Papa, S. (2003) Biochemistry, 42, 4607–4612.
Traber, R., Kramer, H. E., and Hemmerich, P. (1982) Biochemistry, 21, 1687–1693.
Verkhovsky, M. I., Morgan, J. E., and Wikstrom, M. (1995) Biochemistry, 34, 7483–7491.
Verkhosvsky, M. I., Jasaitis, A., Verkhovskaya, M. L., Morgan, J. E., and Wikstrom, M. (1999) Nature, 400, 480–483.
Michel, H. (1999) Biochemistry, 38, 15129–15140.
Mitchell, R., and Rich, P. R. (1994) Biochim. Biophys. Acta, 1186, 19–26.
Ostermeier, C., Harrenga, A., Ermler, U., and Michel, H. (1997) Proc. Natl. Acad. Sci. USA, 94, 10547–10553.
Proshlyakov, D. A., Pressler, M. A., and Babcock, G. T. (1998) Proc. Natl. Acad. Sci. USA, 95, 8020–8025.
Budiman, K., Kannt, A., Lyubenova, S., Richter, H. O. M., Ludwig, B., Michel, H., and MacMillan, F. (2004) Biochemistry, 43, 11709–11716.
Van Eps, N., Szundi, I., and Einarsdottir, O. (2003) Biochemistry, 42, 5065–5073.
Ruitenberg, M., Kannt, A., Bamberg, E., Ludwig, B., Michel, H., and Fendler, K. (2000) PNAS, 97, 4632–4636.
Verkhosvsky, M. I., Tuukkanen, A., Backgren, C., Puustinen, A., and Wikstrom, M. (2001) Biochemistry, 40, 7077–7083.
Ruitenberg, M., Kannt, A., Bamberg, E., Fendler, K., and Michel, H. (2002) Nature, 417, 99–102.
Rich, P. R. (1995) Aust. J. Plant. Physiol., 22, 479–486.
Author information
Authors and Affiliations
Additional information
Translated from Biokhimiya, Vol. 70, No. 2, 2005, pp. 220–230.
Original Russian Text Copyright © 2005 by Papa.
Rights and permissions
About this article
Cite this article
Papa, S. Role of cooperative H+/e− Linkage (redox Bohr effect) at heme a/CuA and heme a 3/CuB in the proton pump of cytochrome c oxidase. Biochemistry (Moscow) 70, 178–186 (2005). https://doi.org/10.1007/s10541-005-0099-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10541-005-0099-y