Abstract
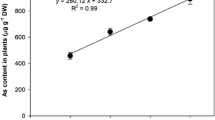
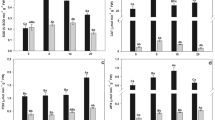
Nitric oxide (NO) is an important molecule involved in the perception of stress induced by toxic compounds such as arsenic (As). The present study investigated the role of NO applied as sodium nitroprusside (SNP) in cell signalling and the ability of NO to attenuate the toxic effects of As (in the form of sodium arsenate) in water hyacinth (Eichhornia crassipes). Water hyacinth plants were collected and assigned to one of the following treatments: control; 100 μM SNP; 20 μM As; or 20 μM As + 100 μM SNP. The plants remained under these conditions for 0, 4, 12, and 24 h. After each time interval, the plants were collected and As absorption, production of reactive oxygen species (ROS), integrity of membranes, and antioxidant enzyme activities were evaluated. The plants were able to absorb and accumulate large amounts of As, even after only four hours of exposure to the pollutant. The absorption and bioaccumulation factor of As was even greater when plants were exposed to both As and SNP. The accumulation of As triggered increases in ROS production and cell membrane damage. In the presence of SNP, the tolerance index to As increased and damage was mitigated. Therefore, from the present work, it was possible to conclude that exogenous NO influenced the ability of plants to tolerate As; this finding has implications for phytoremediation in areas contaminated by As.
Similar content being viewed by others
Abbreviations
- CAT:
-
catalase
- MDA:
-
malondialdehyde
- POX:
-
peroxidase
- ROS:
-
reactive oxygen species
- SNP:
-
sodium nitropruside
- SOD:
-
superoxide dismutase
- TI:
-
tolerance index
References
Anderson, M.D., Prasad, T.K., Stewart, C.R.: Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotylus of maize seedlings. — Plant Physiol. 109: 1247–1257, 1995.
Arasimowicz, M., Floryszak-Wieczorek, J.: Nitric oxide as a bioactive signaling molecule in plant stress responses. — Plant Sci. 172: 876–887, 2007.
Beauchamp, C., Fridovich, I.: Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. — Anal. Biochem. 44: 276–287, 1971.
Boveris, A., Alvarez, S., Bustamante, J., Valdez, L.: Measurement of superoxide radical and hydrogen peroxide production in isolated cells and subcellular organelles. — Methods Enzymol. 349: 280–287, 2002.
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. — Anal. Biochem. 72: 248–254, 1976.
Chance, B., Maehley, A.C.: Assay of catalases and peroxidases. — Method. Enzymol. 2: 764–775, 1955.
Clark, R.B.: Characterization of phosphatase of intact maize roots. — J. Agr. Food Chem. 23: 458–460, 1975.
Crow, J.P.: Peroxynitrite scavenging by metalloporphyrins and thiolates. — Free Radicals Biol. Med. 28: 1487–1494, 2000.
Farnese, F.S., Oliveira, J.A., Gusman, G.S., Leao, G.A., Ribeiro, C., Siman, LI., Cambraia, J.: Plant responses to arsenic: the role of nitric oxide. — Water Air Soil Pollut. 224: 1660–1667, 2013.
Farnese, F.S., Oliveira, J.A., Gusman, G.S., Leão, G.A., Silveira, N.M., Silva, P.M., Ribeiro, C., Cambraia, J.: Effects of adding nitroprusside on arsenic stressed response of Pistia stratiotes L. under hydroponic conditions. — Int. J. Phytorem. 16: 123–137, 2014a.
Farnese, F.S., Oliveira, J.A., Lima, F.S., Leão, G.A., Gusman, G.S., Silva, L.C.: Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. — Braz. J. Biol. 74: 25–31, 2014b.
Gay, C., Gebicki, J.M.: A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. — Anal. Biochem. 284: 217–220, 2000.
Gebicka, L., Didik, J.: Catalytic scavenging of peroxynitrite by catalase. — J. inorg. Biochem. 103: 1375–1379, 2009.
Giannopolitis, C.N., Ries, S.K.: Superoxide dismutases.: I. Occurrence in higher plants. — Plant Physiol. 59: 309–314, 1977.
Gusman, G.S., Oliveira, J.A., Farnese, F.S., Cambraia, J.: Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. — Acta Physiol. Plant. 35: 1201–1209, 2013.
Havir, E.A., McHale, N.A.: Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. — Plant Physiol. 84: 450–455, 1987.
Hayat, S., Hasan, S.A., Mori, M., Fariduddin, Q., Ahmad, A.: Nitric oxide: chemistry, biosynthesis, and physiological role. — In: Hayat S, Mori M, Pichtel J, Ahmad A. (ed.): — Nitric Oxide in Plant Physiology. Pp. 15–21. Wiley, Weinheim 2010.
Heath, R.L., Packer, L.: Photoperoxidation in isolated chloroplast. I. Kinetics and stoichometry of fatty acid peroxidation. — Arch. Biochem. Biophys. 125: 189–198, 1968.
Hermes, V.S., Dall’Asta, P., Amaral, F.P., Anacleto, K.B., Arisi, A.C.M.: The regulation of transcription of genes related to oxidative stress and glutathione synthesis in Zea mays leaves by nitric oxide. — Biol. Plant. 57: 620–626, 2013.
Huang, R.Q., Gao, S.F., Wang, W.J., Staunton, S., Wang, G.: Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. — Sci. total Environ. 368: 531–541, 2006.
Iqbal, M.T.: Mitigation of arsenic by water hyacinths (Eichhornia crassipes) plant. — Asian J. Water Environ. Pollut. 8: 9–14, 2011.
Ismail, G.S.M.: Protective role of nitric oxide against arsenicinduced damages in germinating mung bean seeds. — Acta Physiol. Plant. 34: 1303–1311, 2012.
Kuo, M.C., Kao, C.H.: Aluminium effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. — Biol. Plant. 46: 149–152, 2003.
Leão, G.A., Oliveira, J.A., Farnese, F.S., Gusman, G.S., Felipe, R.T.A.: Sulfur metabolism: different tolerances of two aquatic macrophytes exposed to arsenic. — Ecotoxicol. Environ. Safety 105: 36–42, 2014a.
Leão, G.A., Oliveira, J.A., Felipe, R.T.A., Farnese, F.S., Gusman, G.S.: Anthocyanins, thiols, and antioxidant scavenging enzymes are involved in Lemna gibba tolerance to arsenic. — J. Plant Interact. 9: 143–151, 2014b.
Litter, M.I., Alarcón-Herrera, M.T., Arenas, M.J., Armienta, M.A., Avilés, M., Cáceres, R.E., Cipriani, H.N., Cornejo, L., Dias, L.E., Cirelli, A.F., Farfán, E.M., Garrido, S., Lorenzo, L., Morgada, M.E., Olmos-Márquez, M.A., Pérez-Carrera, A.: Small-scale and household methods to remove arsenic from water for drinking purposes in Latin America. — Sci. total Environ. 429: 107–122, 2011.
Marin, A.R., Pezeshki, S.R., Masschenlyn, P.H., Choi, H.S.: Effect of dimethylarsenic acid (DMAA) on growth tissue arsenic and photosynthesis in rice plants. — J. Plant Nutr. 16: 865–880, 1993.
Mascher, R., Lippman, B., Holzinger, S., Bergmann, H.: Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. — Plant Sci. 163: 961–969, 2002.
Meharg, A.A., Hartley-Whitaker, J.: Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. — New Phytol. 154: 29–43, 2002.
Miteva, E., Hristova, D., Nenova, V., Manev, S.: Arsenic as a factor affecting virus infection in tomato plants: changes in plant growth, peroxidase activity and chloroplast pigments. — Sci. Hort. 105: 343–358, 2005.
Mohammadi, M., Karr, A.L.: Superoxide anion generation in effective and ineffective soybean root nodules. — J. Plant Physiol. 158: 1023–1029, 2001.
Nakano, Y., Asada, K.: Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. — Plant Cell Physiol. 22: 867–880, 1981.
Peixoto, P.H.P., Cambraia, J., Sant’ana, R., Mosquim, P.R., Moreira, M.A.: Aluminum effects on lipid peroxidation and on activities of enzymes of oxidative metabolism in sorghum. — Rev. Bras. Fisiol. Veg. 11: 137–143, 1999.
Shah, K., Nongkynrih, J.M.: Metal hyperaccumulation and bioremediation. — Biol. Plant. 51: 618–634, 2007.
Shaibur, M.R., Kitajima, N., Imamul-Huq, SM., Kawai, S.: Arsenic-iron interaction: Effect of additional iron on arsenic-induced chlorosis in barley grown in water culture. — Soil Sci. Plant Nutr. 55: 739–746, 2009.
Shri, M., Kumar, S., Chakrabarty, D., Trivedi, P.K., Mallick, S., Misra, P., Shukla, D., Mishra, S., Srivastava, S., Tripathi, R.D., Tuli, R.: Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. — Ecotoxicol. Environ. Safety 72: 1102–1110, 2009.
Singh, H.P., Kaur, S., Batish, D.R., Sharma, V.P., Sharma, N., Kohli, R.K.: Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). — Nitric Oxide 20: 289–297, 2009.
Wilkins, D.A.: The measurement of tolerance to edaphic factors by means of root growth. — New Phytol. 80: 623–633, 1978.
Xiong, J., Fu, G., Tao, L., Zhu, C.: Roles of nitric oxide in alleviating heavy metal toxicity in plants. — Arch. Biochem. Biophys. 497: 13–20, 2010.
Yamamoto, Y., Kobayashi, Y., Matsumoto, H.: Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. — Plant Physiol. 125: 199–208, 2001.
Zhang, Z.C., Qiu, B.S.: Reactive oxygen species metabolism during the cadmium hyperaccumulation of a new hyperaccumulator Sedum alfredii (Crassulaceae). — J. Environ. Sci. 19: 1311–1317, 2007.
Zhao, F.J., Ma, J.F., Meharg, A.A., McGrath, S.P.: Arsenic uptake and metabolism in plants. — New Phytol. 181: 777–791, 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgments: The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Federal University of Viçosa for financial support.
Rights and permissions
About this article
Cite this article
Andrade, H.M., Oliveira, J.A., Farnese, F.S. et al. Arsenic toxicity: cell signalling and the attenuating effect of nitric oxide in Eichhornia crassipes . Biol Plant 60, 173–180 (2016). https://doi.org/10.1007/s10535-015-0572-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-015-0572-4