Abstract
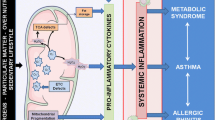
Over several decades, asthma has evolved from being recognized as a single disease to include a diverse group of phenotypes with dissimilar natural histories, pathophysiologies, responses to treatment, and distinctive molecular pathways. With the application of Occam’s razor to asthma, it is proposed that there is one cause underlying the numerous phenotypes of this disease and that the responsible molecular pathway is a deficiency of iron in the lung tissues. This deficiency can be either absolute (e.g. asthma in the neonate and during both pregnancy and menstruation) or functional (e.g. asthma associated with infections, smoking, and obesity). Comparable associations between asthma co-morbidity (e.g. eczema, urticaria, restless leg syndrome, and pulmonary hypertension) with iron deficiency support such a shared mechanistic pathway. Therapies directed at asthma demonstrate a capacity to impact iron homeostasis, further strengthening the relationship. Finally, pathophysiologic events producing asthma, including inflammation, increases in Th2 cells, and muscle contraction, can correlate with iron availability. Recognition of a potential association between asthma and an absolute and/or functional iron deficiency suggests specific therapeutic interventions including inhaled iron.
Similar content being viewed by others
Introduction
Asthma is a disorder characterized by (1) recurring symptoms of wheezing, chest tightness, and/or shortness of breath, (2) pathologic evidence of airway inflammation, and (3) physiologic changes in bronchial hyperresponsiveness and airflow obstruction. It is the most common chronic illness in childhood and a major cause of morbidity in adults, affecting 4–17 % of children and 7–10 % of adults in the United States and, overall, nearly 30 million Americans (Yun et al. 2012).
Over several decades, asthma has evolved from being recognized as a single disease to include a diverse group of phenotypes with dissimilar natural histories, pathophysiologies, and responses to treatment (Gauthier et al. 2015; Haldar et al. 2008). It is assumed that there are distinctive molecular pathways underlying these disparate conditions, but they remain to be defined.
To provide simplicity, one can extrapolate Occam’s razor to asthma and propose that there is one cause underlying the numerous phenotypes of this disease and that the responsible molecular pathway is a deficiency of iron, absolute and/or functional, in the lung tissues. In this review, iron homeostasis in asthmatic conditions is delineated. Associations between iron homeostasis and (1) recognized determinants of asthma (Table 1), (2) co-morbidity of asthma, and (3) therapeutic interventions in asthma are demonstrated. Finally, possible relationships between the pathophysiologic events producing asthma and iron homeostasis are described.
Iron homeostasis
As a result of a favorable oxidation–reduction potential and its abundance in nature, iron performs a wide range of biological functions. This metal is therefore an essential micronutrient required for virtually every aspect of normal cell function, and living systems must have iron to survive. However, water-soluble ferrous ion (Fe2+) was effectively removed from the Earth’s crust after its reaction with atmospheric oxygen produced by photosynthesis. Ferric ion (Fe3+) remained but at concentrations inadequate to meet the requirements for life; the concentrations of Fe3+ soluble in water at physiologic pH values is about 10−18 M while that required for life approaches 10−6 M. To support life, greater quantities of metal were needed. This challenge to living systems was achieved by (1) the chemical reduction of Fe3+ to Fe2+ (i.e. ferrireduction) with its subsequent import and utilization and (2) the complexation of Fe3+ with chelators coupled to receptors for uptake of the complex, which rendered the metal bioavailable. In addition to solubility-limited availability, the metal presents a potential for oxidative stress related to iron-catalyzed generation of radicals. Such reactivity mandates that iron availability be tightly regulated and, as a result, life normally exists at the interface of iron-deficiency and iron-sufficiency.
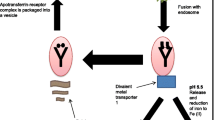
Cellular iron homeostasis is maintained by a coordinated expression of proteins involved in the import, export, storage, and utilization of this metal (Hentze et al. 2004). Post-transcriptional control mediated by iron-regulatory proteins (IRP) is essential (Wallander et al. 2006). IRP1, the cytosolic counterpart of mitochondrial aconitase, is a bifunctional protein that, through [4Fe–4S] cluster assembly/disassembly, shifts from the aconitase to the IRP1 form in response to decreased intracellular iron concentrations (Kennedy et al. 1992). Accordingly, iron levels regulate the RNA-binding capacity of IRP1. With diminished availability of the metal, IRP1 binds to cis-acting mRNA moieties termed iron-responsive elements to impact the expression of proteins involved in import, export, storage, and utilization. Mechanisms include stabilization of the mRNA of the divalent metal transport 1 importer (DMT1) and transferrin receptor 1 to promote translation and increase their expression while also suppressing the synthesis of the storage protein ferritin and the exporter ferroportin-1 (Mims and Prchal 2005).
The serum ferritin level is currently accepted as the best indicator of stored and total body iron (Kruger et al. 2012). It is assumed that there is an association between the total body iron and that concentration of metal available to cells, tissues, and living systems. Normally, concentrations of iron inadequate to support the function of cells, tissues, and living systems are reflected by levels of serum ferritin, serum iron, transferrin, transferrin receptor, and other proteins involved in iron homeostasis. Insufficient iron can result from both an absolute and a functional deficiency of the metal (Lopez et al. 2015). In an absolute deficiency, iron concentrations are lacking as a result of either decreased intake or an elevated loss; provision of the metal can improve such a condition. A functional deficiency frequently develops from the introduction of an inappropriate chelator that binds host iron, decreasing concentrations available to the cell (e.g. a particle) (Ghio et al. 2013). In the presence of such an inappropriate chelator, a functional deficiency may exist despite total iron being either normal or even elevated.
The host response to a deficiency in iron (both absolute and functional) includes an increased expression of importers in an attempt by affected cells to reverse the loss of requisite metal. Concomitantly, a disruption of iron homeostasis with metal deficiency in a living system necessitates prioritization of the available iron with potential biochemical and physiological consequences (Guiang et al. 1997). With deficiency, the distribution of the metal can favor specific tissues with red blood cells possibly assuming priority over others. Mechanisms by which prioritization of iron delivery to tissues is regulated have not been fully delineated. Iron concentrations in the non-heme tissues (e.g. skeletal muscle and heart) can subsequently appear to be sacrificed at the expense of red cell iron incorporation (McKay et al. 1983; Zamora et al. 2016). Such a propensity of red blood cells to accumulate iron in preference to other tissues may reflect that 80–90 % of the body’s TfRs in the human normally reside on erythroid cells (Beguin 1992). However, blood hematocrit can also decrease before reductions in brain iron suggesting that, at least in older animals, brain iron can be spared at the expense of the erythron (Dallman et al. 1975). Accordingly, while anemia can reflect iron deficiency in all tissues of a living system, metal concentrations in peripheral tissues can be diminished prior to any loss in red blood cells. It can be assumed that if there is anemia present, the risk for iron deficiency in tissues other than red blood cells may be increased.
Iron concentrations in serum samples from asthmatics
Measurement of indices of iron homeostasis in serum is the most direct manner of demonstrating metal deficiency in asthma. Serum iron concentrations demonstrated no differences in comparisons between healthy subjects and asthmatic patients (Vural et al. 2000). Plasma iron levels were also reported to be elevated in asthmatics (Ekmekci et al. 2004; Kocyigit et al. 2004). More recently, using data from the 2007 to 2010 National Health and Nutrition Examination Survey (NHANES) including serum ferritin, serum soluble TfR, and TfR/log ferritin, and iron stores among women (aged 20–49 years) in the United States, indices of iron homeostasis were associated with asthma (Brigham et al. 2015). Specifically, serum ferritin greater than 76 ng/mL was associated with decreased odds of lifetime asthma, of current asthma and of asthma attack/episode in the past year. In addition, increases in tissue iron need and decreases in body iron, (represented by lower sTfR and lower sTfR/log ferritin, respectively) correlated with decreases in forced expiratory volume in one second, supporting an influence of tissue and body iron on lung function.
Demographic determinants of asthma
Age and gender
The incidence and prevalence of asthma demonstrate an inverse relationship with age; both are greatest in young children and less in late adolescence and adulthood (Dodge and Burrows 1980; Singleton et al. 2006). More than 50 % of patients with childhood asthma enter clinical remission by puberty. Regarding the relationship between total body iron and age, the healthy newborn has relatively high concentrations of stored iron. During the first few months of life, when milk (which is low in iron) is the primary source of nutrition, the stockpiled metal supports growth of the newborn. Subsequently, iron stores are mobilized after birth and decrease with duration of nursing. With the introduction of iron-rich solid foods, the concentration of total metal concentration increases, and an invariable accumulation of iron continues through life (Fig. 1). The observed incidence and prevalence of asthma demonstrate an inverse relationship with total iron concentrations, supporting the relationship between asthma and iron deficiency. The remission of asthma with aging can reflect augmented iron availability as stored concentrations (e.g. serum ferritin) increase with age, and deficiency decreases. Comparable to measures of asthma incidence and prevalence, bronchial hyperresponsiveness decreases with age again reflecting elevated metal concentrations among older individuals (Burrows et al. 1995; Le Souef et al. 1995).
Similar to age, gender is a major determinant of both asthma and iron status. Males can have a higher prevalence of asthma in infancy and early childhood (Almqvist et al. 2008; Schatz and Camargo 2003); asthma is diagnosed 1.5 times more frequently in young boys than in young girls (Dodge and Burrows 1980). Regarding hospital admissions for asthma, males are admitted for asthma nearly twice as often as age-identical females in the 0- to 5-year-old and 6- to 10-year-old age groups (Skobeloff et al. 1992). With the onset of puberty, the prevalence of asthma becomes higher in females and will remain higher throughout the remainder of life (Dodge and Burrows 1980; Melgert et al. 2007; Postma 2007). While the prevalence of asthma in boys (7.7 %) was similar to girls (7.4 %) at 11.1 years of age, the prevalence was 6.2 and 4.3 % in females and males respectively at 16.3 years of age (Vink et al. 2010). Similar to prevalence, the rates of emergency visits, hospitalizations, and health care utilization for asthma are higher in adult females than males (Debley et al. 2004; Lee et al. 2006). For hospital admissions, the female-to-male ratio is nearly 3:1 among those between 20 and 50 years of age, while females over 50 years of age are admitted for asthma at a rate of about 2.5:1 when compared with their age-equivalent male counterparts (Skobeloff et al. 1992). Similarly, there is evidence suggesting that airway hyperreactivity is more prevalent in women than in men in the general population (Leynaert et al. 1997; Manfreda et al. 2001).
These disparities in asthma measures between the genders correspond to the observation that serum ferritin levels in males up to 11 years of age are lower than in females, reflecting less total iron in males (Emond et al. 1996; Milman and Kaas Ibsen 1984) (Fig. 1). Following puberty and throughout adulthood, females will have lower iron concentrations (Fig. 1). These lower concentrations of stored iron among females correlate with the disparities between the genders in asthma incidence/prevalence and measures of severity, reinforcing the proposed relationship of asthma with iron homeostasis.
Ethnicity
Disparities in the incidence and prevalence, indices of morbidity and mortality, and treatment response of asthma have been identified among ethnic groups (Drake et al. 2008). Asthmatics with African-American ancestry have particularly severe disease (Oliveti et al. 1996; Rumpel et al. 2012). It has been proposed that these differences reflect dissimilarities in smoking, exposures to environmental tobacco smoke and air pollution, compliance with and response to therapies, urbanization, psychosocial stress, economic and educational advantages, and availability of health insurance. However, corresponding to an increased risk for asthma, African-American ethnicity has been associated with alterations in iron homeostasis (Li et al. 2015; McLaren et al. 2012). Diminished body and serum iron levels have been observed among African-Americans, supporting the inverse association between asthma and metal availability (Chambers et al. 2006; Pfeiffer et al. 2013).
Physiologic determinants of asthma
Prenatal and perinatal factors
Asthma diagnoses are increased among newborns with low birth weight and those born preterm (Been et al. 2014; Mu et al. 2014). Size at birth is associated with the occurrence of asthma not only in the neonate but also in later life (Barker et al. 2013; Gregory et al. 1999; Lu et al. 2012). A gender difference has been observed in the effect of birthweight on the prevalence of asthma in childhood and even among adults, with females showing a disproportionate effect (Brostrom et al. 2013). Similar to birthweight, preterm delivery predicts asthma with an approximately fourfold increase in the incidence for individuals born prematurely (Beaudoin et al. 2013; Landry et al. 2011). A negative dose–response association has been noted between gestational age and the purchase of prescription asthma medication in infancy and childhood (Damgaard et al. 2015). In another study, children born preterm were observed to have a higher risk of asthma compared to term children at ages 0–5 and 6–9 years (He et al. 2015). Preterm babies also have a higher prevalence of abnormally low pulmonary function and bronchial hyperresponsiveness at 21 or 22 years of age (Islam et al. 2015; Landry et al. 2016; Vollsaeter et al. 2015).
Increased risks for asthma among those born preterm and with low birth weight correspond to changes in iron homeostasis and deficiency of the metal. The requirement for iron during pregnancy exceeds that in the non-pregnant state and increases through gravidity approximating 0.8, 4–5, and >6 mg in the first, second, and third trimesters respectively (Bothwell 2000; Cetin et al. 2011). The additional metal is used for (1) expansion of the maternal erythrocyte mass, (2) replacement of maternal blood lost at delivery, (3) creation of placenta, and (4) fetal growth. In later pregnancy, the amount of iron that can be absorbed from the diet is frequently less than that needed. Therefore, a woman must enter pregnancy with iron stores in excess of 300 mg if requirements of the fetus are to be met. A minority of women may have this reserve (Baker et al. 2010). Reflecting this lack of reserve, maternal serum ferritin concentration decreases during pregnancy (Puolakka 1980); in one study, serum ferritin decreased from 31.9 ng/mL in the first trimester to 10.3 ng/mL in the third trimester (Shields et al. 2011). Subsequently, indices of iron homeostasis in the pregnant woman (e.g. hemoglobin levels, transferrin saturation, and serum ferritin concentrations) reflect reduced stores. Non-hereditary, non-hemolytic anemia is the most common complication of pregnancy with rates around 9.3 % (Bruce et al. 2008). However, among low-income minority populations in the United States, iron-deficiency anemia can affect approximately 27 % of pregnancies in the third trimester (American College of Obstetricians and Gynecologists 2008; Scholl 2005).
The fetus normally stockpiles relatively high concentrations of iron acquired from the mother; this transfer is especially effective during the last trimester of the pregnancy (Fig. 2). As a result of the requirement for iron transfer, variability in maternal stores will influence that of the newborn infant, the child, and perhaps the adult (Kaneshige 1981; Puolakka et al. 1980). Maternal serum iron and fetal iron levels positively correlate suggesting that the fetus can extract iron in amounts proportional to the levels available in the mother (Hokama 1999; Singla et al. 1979). Fetal iron reserves are dependent on maternal iron reserves and, in turn, the mother’s iron intake (Kelly et al. 1978; Milman et al. 1991; Takala et al. 2010). This correlation between indices of maternal and newborn iron status reveals a considerable vulnerability of the fetus to iron deficiency during intrauterine life. With limited stores, a lack of a sufficient dietary intake of the metal, and an inability to regulate absorption by the gastrointestinal tract, iron deficiency can result (Collard 2009). Subsequently, maternal anemia will be a major contributor to both iron deficiency and anemia among infants (Liu et al. 2015).
Along with anemia, low birth weight, and preterm birth, asthma can be included in the spectrum of disease associated with maternal-fetal iron deficiency (Fig. 3). A birth cohort study found an inverse association between umbilical cord iron levels and later onset wheeze (Shaheen et al. 2004). Maternal anemia during pregnancy, reflecting diminished iron availability in both the mother and the fetus, was associated with wheeze and asthma in children (Triche et al. 2011). Reduced maternal iron status during pregnancy (even as early as in the first trimester) was directly related to increased wheeze and atopic sensitization and decreased lung function in children up to 10 years of age (Nwaru et al. 2014).
Regarding anemia in the neonate, term newborns demonstrate a decline in hemoglobin after birth reflecting inadequate quantities of iron (Ferri et al. 2014). In infants, iron deficiency anemia most frequently occurs 3 months after iron stores are depleted, which is typically at 6–9 months of age (Shakur et al. 2010).
Iron is an absolute requirement for cell proliferation, and its deficiency leads to apoptosis/cell death (Hileti et al. 1995; Yu et al. 2007). Comparable to cultured cells, iron stimulates growth in living systems including humans. Beginning pregnancy with non-depleted iron stores increases infant birthweight while maternal anemia diminishes birthweight (Levy et al. 2005; Ribot et al. 2012; Singla et al. 1997). Mild to moderate iron deficiency in the mother, even without anemia, contributes to lower iron reserves in the fetus and to lower birth weight (Bhargava et al. 1991). Birth weight is also associated with decreased levels of iron as indicated not only by maternal hemoglobin and hematocrit but also by ferritin (Rasmussen 2001). Newborns of non-anemic mothers supplemented with iron demonstrate greater birthweights (and elevated serum ferritin concentrations) (Aranda et al. 2011; Wise 2013). Small for gestational age infants have lesser total iron stores (blood ferritin) as compared to appropriate for age infants at birth (Mukhopadhyay et al. 2011). Birth weight can significantly correlate with the hemoglobin concentration at 6 months of age (Gunnarsson et al. 2004; Shakur et al. 2010). Greater doses of iron supplementation impact higher birth weights in newborns (Ribot et al. 2013). The associations of low birth weight with asthma likely reflect a relationship of both with concentrations of available iron.
The understanding of the biological mechanisms that control the timing of delivery is incomplete. Thus the relationship of preterm delivery with iron homeostasis is poorly understood. In both the mother and the fetus, decreased indices of iron availability correspond with preterm delivery. Maternal anemia is associated with a shorter gestation period and it is plausible that the frequently observed association between maternal hemoglobin/hematocrit with birth weight are caused by a shorter gestation (Allen 2001; Gunnarsson et al. 2004; Shakur et al. 2010). Ferritin concentrations are positively associated with gestation duration with iron supplementation effecting longer pregnancies (Hemminki and Rimpela 1991; Hemminki and Starfield 1978). Plasma iron concentrations measured in the umbilical cord blood of babies rise with gestational age (Scott et al. 1975). Similarly, fetal iron stores reflected by umbilical cord serum ferritin can almost triple from 63 to 171 µg/L between 23 and 41 weeks gestation (Siddappa et al. 2007) (Fig. 2). Premature birth is similarly associated with iron deficiency in the first 6 months of life, and iron supplements can reduce this risk (Gorten and Cross 1964; Lundstrom et al. 1977; Uijterschout et al. 2015) (Fig. 2). With higher doses of iron supplementation, there is less iron deficiency anemia and fewer preterm deliveries and low birth weights in newborns (Ribot et al. 2013). Reflecting iron stores, mean hemoglobin in preterm infants was lower than mean hemoglobin in full-term infants with values of 13.4, 14.5, 15.0, and 16.8 g/dL at 26–30, 28, 32, and 37–40 weeks gestational age, respectively. Similarly, premature children demonstrate lower levels of ferritin (Takala et al. 2010). The correlation of premature delivery to asthma likely reflects an association of both with available iron.
Diurnal variation
Nocturnal worsening of symptoms of asthma (e.g. dyspnea and cough) is frequent and can be accompanied by elevations in airway inflammation, airflow limitation, and airways hyperresponsiveness (Spengler and Shea 2000; Sutherland 2005). As many as 75 % of asthmatic subjects are awakened by asthma symptoms at least once per week with approximately 40 % experiencing nocturnal symptoms on a nightly basis. Both healthy and asthmatic individuals show the lowest levels of forced expiratory volume in one second at approximately 4 AM, but the latter demonstrate increased circadian variations in pulmonary function indices. Most deaths related to asthma symptoms occur during the night (Tough et al. 1998). Nocturnal asthma has been attributed to allergen exposure in the bedroom, gastroesophageal reflux, a drop in body temperature with airway cooling, and a diminished efficacy of medications. However, human serum iron levels also demonstrate diurnal variation with nadirs in availability being in the early morning. Thus serum iron levels parallel the increase in asthma symptoms, physiologic changes, and deaths (Cao et al. 2012; Ridefelt et al. 2010). Tissue iron levels in animal models reveal a comparable diurnal variation further supporting a participation of iron homeostasis in this form of asthma (Unger et al. 2009; Unger et al. 2014).
Menstruation
Peri-menstrual worsening of asthma has been documented in a significant number of asthmatic women. It is more commonly observed in severe disease and has been correlated with both aspirin sensitivity and lower baseline pulmonary function (Eliasson et al. 1986; Rao et al. 2013; Vrieze et al. 2003). Menstruation can function as a contributing factor in the development of episodes of near fatal asthma with significantly more near fatal asthma episodes observed on the first day of menstruation than on the remaining days (Martinez-Moragon et al. 2004). Changes in pulmonary function and bronchial hyperreactivity show associations with the menstrual cycle in both healthy and asthmatic populations (Dratva et al. 2010; Farha et al. 2009; Jeon et al. 2009). Supporting an affiliation of peri-menstrual worsening of asthma with decreased iron availability, women 18–44 years of age with regular menstrual cycles showed cyclical changes in serum concentrations of hemoglobin, iron, and ferritin as well as transferrin saturation with the lowest values observed during menses (Kim et al. 1993).
Pregnancy
Asthma is a common condition during pregnancy. Worldwide the prevalence of asthma among pregnant women is on the rise, and pregnancy leads to a worsening of asthma for many (Murphy and Gibson 2011). Asthma control is most frequently impacted in the latter stages of pregnancy. Despite iron absorption rising in pregnancy, the amount that can be absorbed from the diet is frequently less than the iron requirements (Bothwell 2000). The serum ferritin concentration drops during pregnancy reflecting mobilization of iron from maternal stores to meet the increased demands of the fetus. In addition, maternal asthma is associated with poor pregnancy outcomes including increased risks for low birthweight and preterm delivery. Iron deficiency can be seen as the common risk factor leading to all these outcomes (Demissie et al. 1998; Murphy et al. 2011).
Exercise
Exercise-induced bronchoconstriction is airway hyperresponsiveness with exercise and occurs frequently among athletes even in the absence of a diagnosis of asthma or any respiratory symptoms. In general, exercise-induced bronchoconstriction affects 8–17 % of the population of individuals without asthma, 30–70 % in elite athletes, and up to 90 % in the population of individuals with asthma (Anderson 2012; Burr et al. 2006; Randolph 2009; Weiler et al. 2007). Airway hyperresponsiveness is more common among athletes relative to those individuals in the general population (Langdeau et al. 2000; Sue-Chu 2012). Exercise-induced bronchoconstriction is often the first manifestation of asthma; there are a significant number of individuals with no history of asthma that respond to vigorous exercise with a mild degree of exercise-induced bronchoconstriction (Godfrey and Bar-Yishay 1993). Exercise-induced asthma was initially described in cross-country skiers, but studies demonstrate that asthma is more common in all trained athletes relative to control subjects (Helenius et al. 1998). Exercise-induced asthma is especially common in long-distance runners and elite swimmers (Helenius et al. 1997).
The increased prevalence of asthma among athletes has been attributed both to an intense respiratory heat and water exchange and to an increased penetration of allergens and pollutants into the lower airways (Boulet and O'Byrne 2015). Exercise is also associated with an acute decrease in serum iron and chronic reductions in iron stores manifested by a “sports anemia”. With exercise a decrement in iron concentration can occur quickly in athletes with serum iron falling 45 % within 15 min of the completion of an ironman triathlon race, 46 % following a 12-h cross-country ski race, and 39 % following a 24-h race (Ginsburg et al. 1996; Pattini et al. 1990). Among untrained individuals, serum iron similarly decreases within 24 h after a high-intensity exercise bout (Smith and Roberts 1994). With repeated aerobic exercise, there can be changes in many measures of iron homeostasis including serum iron, ferritin, transferrin, and transferrin saturation (Auersperger et al. 2013; Di Santolo et al. 2008; Mainous and Diaz 2009; Wilkinson et al. 2002). Accordingly, athletes have a greater risk of iron depletion and anemia; this is especially true for females (Fallon, 2004; Landahl et al. 2005; Ostojic and Ahmetovic 2008; Reinke et al. 2012). Nearly one-fifth of recreational athletes have anemia and a third have an iron deficit (Di Santolo et al. 2008). Changes in iron homeostasis are considered a result of hemodilution, increased destruction of erythrocytes, depressed iron absorption, and increased iron loss in sweat and through the gastrointestinal tract. Comparable to other phenotypes, exercise-induced asthma corresponds with decrements in concentrations of stored and available iron.
Pathologic determinants of asthma
Infections
Respiratory infections are considered to both impact the development of asthma and worsen existing asthma. Respiratory tract infections caused by the viruses, Chlamydophila and Mycoplasma have been postulated to have significant roles in the pathogenesis of asthma (Busse et al. 2010; Guilbert and Denlinger 2010). For patients either at risk of asthma or with existing asthma, viral respiratory tract infections can affect the disease. Studies also invoke atypical bacterial infections, Mycoplasma pneumoniae and Chlamydia pneumoniae, as factors in both acute exacerbation and chronic asthma (Martin 2006). New evidence has shown that wheezing episodes early in life due to human rhinoviruses are a major risk factor for the diagnosis of asthma at age 6 years. Viral respiratory tract infections, especially those caused by human rhinoviruses, are associated with asthma exacerbations. Symptoms can persist for up to 3 months following an acute infection. Respiratory viruses are associated not only with asthmatic exacerbations but also with the response to methacholine challenge in both humans and animal models (Cheung et al. 1995; Little et al. 1978; Sorkness et al. 1994). Post-viral bronchial hyperreactivity syndrome is a common complication of respiratory tract viral infections (Ostransky and Blais 1991; Xepapadaki et al. 2005).
Infections disrupt iron homeostasis in cells, animal models, and humans. In cells, infection promotes an iron deficiency (Fillebeen and Pantopoulos 2013). In animal models, serum iron concentrations decrease following infection reflecting a diminished metal availability (Brosnahan et al. 2012; Heegaard et al. 2000; Konijn and Hershko 1977). Similarly, infections in humans impact iron homeostasis with elevations in serum ferritin and TfR while serum iron decreases (Butensky James et al. 2009; Kim et al. 2013). Decrements in serum iron may be one of the earliest responses to infection in humans (Hoppe and Hulthen 2007). Comparable to levels in the serum, iron availability in the lung is predicted to diminish with infection, and it is proposed that this decrease leads to asthmatic exacerbation. While less studied, this disruption in iron homeostasis is observed following viral infections. Iron is needed for viral replication. Therefore, by ensuring the infected cell is iron replete, a virus favors its own growth (Drakesmith and Prentice 2008). Conversely, reducing iron availability to viruses should benefit the host. Such a reaction has been documented with lower serum iron, percent transferrin saturation, hemoglobin, and hematocrit with mumps and chickenpox virus infections (Cemeroglu and Ozsoylu 1994; Weinberg 1996). This hypoferremia following virus infections is expected to increase the risk for asthma.
Acute phase response
During an acute infection, controlling the initial proliferation and dissemination of microbials can be critical in determining the outcome for the host. Decreasing concentrations of requisite iron available to the pathogen is an effective host defense mechanism employed to limit such replication. Hepcidin coordinates this decreased metal availability by binding to ferroportin and triggering internalization and degradation of the exporter. This results in decreased iron uptake by enterocytes and reduced metal transport by macrophages (Schmidt 2015). Accordingly, during infection serum iron concentrations can be reduced to about 30 % of normal, a physiological change known as the hypoferremia of infection, which limits iron availability for microbes but also leads to increased iron storage in macrophages of the reticuloendothelial system. This same pathway participates in the inflammatory response to numerous agents and stressors (e.g. particles, surgery, and autoimmune disease) and is included in the acute phase response. This can result in an anemia of inflammation (both acute and chronic). A purpose of the acute phase response can be to influence iron homeostasis thereby minimizing the challenge to the living system. The acute phase response can generate an iron deficiency with serum iron decreasing to a nadir by 48 h (Solis et al. 2006). Complete recovery of serum iron concentrations to normal may necessitate more than 45 days. A similar evolution is observed in the parameters dependent on iron levels including the red cell count, serum hemoglobin level, and hematocrit. Finally, the acute phase response includes changes in the expression of numerous genes and proteins involved in the iron regulatory pathway not only in the liver but also in extra-hepatic tissues (Sheikh et al. 2007). Subsequently, iron concentrations can be diminished in serum and throughout the body. After intramuscular injection of turpentine to initiate an inflammatory response in an animal model, levels of iron were decreased in extra-hepatic tissues (e.g. brain) (Malik et al. 2011). During this response, TfR-1 increased in the extra-hepatic tissues reflecting a relative deficiency of iron. While the effects of inflammatory stimuli have not been investigated in the lung and airways, it is likely that the acute phase response will trigger a hypoferremia in these tissues also. The relationship between the acute phase response and iron homeostasis can account for the association between non-specific inflammation and asthma. The prolonged duration of the acute phase response can also assist in understanding why recovery from an asthmatic exacerbation can be delayed.
Endotoxin
Endotoxin exposure can increase the risks of wheeze and asthma in children (Rennie et al. 2012). The timing of exposure may be of significance since endotoxin exposure in early life has also been proposed to be protective against developing childhood asthma (Campo et al. 2006). Endotoxin exposure is also associated with increased risk for wheeze and greater asthma severity later in life (Celedon et al. 2007; Perzanowski et al. 2006). Household endotoxin exposures were associated with asthma symptoms, current asthma medication use, and wheezing (Thorne et al. 2005). A recent study demonstrated that elevated endotoxin concentrations in house dust were associated with current wheezing, exercise-induced wheeze, and use of prescription medication for wheezing (Thorne et al. 2015).
Endotoxin alters iron homeostasis in both cells and living systems resulting in a hypoferremia between 6 and 24 h after exposure of animal models and humans (Duvigneau et al. 2008; Kemna et al. 2005; Krijt et al. 2006; Orro et al. 2004; Vernooy et al. 2005; Zhang et al. 2005). Some portion of the decrease in iron, but not the entirety, may reflect an acute phase response following endotoxin exposure (Plessers et al. 2015). This decreased iron availability will lead to an increased risk for asthma and its worsening.
Antibiotics
Cohort studies demonstrate a greater risk of persistent wheeze and asthma in early childhood with prenatal antibiotic treatment (Benn et al. 2002; Jedrychowski et al. 2006). A dose response relationship between the number of antibiotic courses and the risk of either wheeze or asthma has been demonstrated (Jedrychowski et al. 2006; McKeever et al. 2002). In addition, substantial obstructive reactions occur in some asthmatic subjects following the inhalation of specific antibiotics (e.g. gentamicin) (Dally et al. 1978). Such reactions appear to be non-immunological in nature.
Antibiotics impact iron homeostasis. Several antibiotics are recognized to function directly as iron chelators to produce an anti-bacterial effect (Badal et al. 2015; Moon et al. 2013; Sobke et al. 2012). Others appear to trigger changes in the expression of iron transport genes, which then contribute to an anti-microbial effect (Ferrandiz and de la Campa 2014; van Delden et al. 2013). Still other antibiotics display activity against microbes in a manner that has not been defined in detail but depends on interference with the host iron homeostasis (Fiori and Van Dijck 2012; Lounis et al. 2003). The impact of antibiotics on iron homeostasis suggests a participation in metal availability, which influences asthma.
Vaccinations
Vaccinations have been associated with exacerbations of asthma (Hassan et al. 1992). Both hypoferremia and anemia have been shown to follow vaccination (Olivares et al. 1989). In addition an acute phase reaction is evident among vaccinated animals. This reaction includes decreased serum iron and reduced metal availability (Andersen et al. 2012; Stokka et al. 1994). In turn, the hypoferremia, anemia, decreased serum iron, and reduced metal availability after vaccination could affect asthma.
Smoking and environmental tobacco smoke
Smoking is a major contributor to asthma and its exacerbations. Smoking is associated with acute decrements in pulmonary function (Kougias et al. 2013; Miller and Sproule 1966; Sobol et al. 1977). Endpoints (including spirometric indices, airway resistance, and conductance) have revealed worsening after smoking a single cigarette. Furthermore, smoking by itself increases airway hyperreactivity (O’Connor et al. 1989; Sunyer et al. 1997; Tashkin et al. 1992). These acute effects of smoking cigarettes are greater among asthmatic individuals relative to non-asthmatics (Papaioannou et al. 2010).
There is an association of maternal cigarette smoking with asthma in the newborn with maternal smoking increasing the risk for (1) physician-diagnosed asthma during both childhood and adolescence and (2) diminished airway function at the end of the first year of life (Hollams et al. 2014; Lau et al. 2002; Neuman et al. 2012; Xepapadaki et al. 2009). There is also support for a relationship between maternal smoking and the other asthma risk factors of low birth weight and pre-term births (Bjerg et al. 2011; Cox et al. 2013; Prabhu et al. 2010); the loss in birth weight is calculated to be 19 g for each cigarette smoked per day (Adriaanse et al. 1996).
Comparable to smoking, exposure to environmental tobacco smoke increases the incidence of wheeze and asthma in children and young people (Dezateux et al. 1999; James et al. 2005; Stein et al. 1999). Maternal exposure to environmental tobacco smoke during pregnancy has also been implicated in low birth weight, preterm delivery, and asthma of the newborn (Burke et al. 2012; Windham et al. 2000).
The effects of cigarette smoking and ETS (particle-related exposures) on iron availability are consistent with effects of other forms of particulate matter (PM). Functional groups at the particle surface can complex cell metal (e.g. carboxylic and phenolic groups in cigarette smoke particles) (Ghio et al. 2013). Accordingly, cell iron is lost to the particle (Fig. 4). In response to a reduction in intracellular iron, the cell generates superoxide (a ferrireductant which can participate in the uptake of the metal) and upregulates both importers (e.g. DMT1) and storage (i.e. ferritin) proteins in an attempt to reacquire requisite iron. This particle exposure results in an altered iron homeostasis with an absolute accumulation of the metal in the cell but diminished concentrations of available iron. Accordingly, smoking and environmental tobacco smoke impact iron homeostasis to increase asthma.
Iron in the developing fetus is accumulated against a concentration gradient. Despite some capacity to tolerate maternal iron deficiency, many stresses will impact the availability of the metal in the developing fetus. There are statistically significant negative correlations between maternal smoking and infants’ total body iron (Pateva et al. 2015). The number of cigarettes smoked per day correlates negatively with infants’ ferritin; newborn infants of women who smoke during pregnancy have lower iron stores than those of non-smoking mothers. Maternal smoking during pregnancy decreases the ferritin concentration in umbilical cord blood (94 and 163 ng/ml in smoking and non-smoking respectively) and placenta (Chelchowska and Laskowska-Klita 2002). Accordingly, more packs per day and more days of smoking during pregnancy lead to diminished iron available to the fetus, and this can increase asthma risk.
Air pollution
Human exposure to air pollution increases morbidity related to asthma (Friedman et al. 2001). Exposure to ambient air pollution is associated with increased asthma symptoms, exacerbations, and a decline in lung function in children (Gent et al. 2009; Guarnieri and Balmes 2014; O’Connor et al. 2008). Asthmatic exacerbations increase with proximity to point sources of air pollution (Loyo-Berrios et al. 2007; Meng et al. 2007). While measures of both indoor and outdoor PM can correlate with asthmatic worsening, the latter demonstrate a greater effect (Koenig et al. 2005). Air pollution particle exposure correlates with decrements in pulmonary function, and elevations in ambient particle exposure increase bronchodilator use among asthmatics (Rabinovitch et al. 2006; Trenga et al. 2006). In addition to exacerbations of asthma, several studies have associated exposures to air pollution particles with the development of asthma. A prospective birth cohort of 4146 children followed from birth to 2 years of age showed a significant correlation between a physician diagnosis of asthma in the first year of life and ambient air PM levels (Brauer et al. 2002). Corresponding to the biological effects of other particles (e.g. cigarette smoking and ETS), maternal exposure to air pollution particles has also been implicated in low birth weight and preterm delivery (Dadvand et al. 2013; Stieb et al. 2012). Comparable to ambient air PM, there are also studies, which support a role for diesel exhaust particles in causing asthma (Heinrich and Wichmann 2004). Exposure to polyaromatic hydrocarbons in diesel exhaust particles increases the risk for childhood asthma (Karimi et al. 2015). Finally, higher prenatal PM2.5 exposure at mid-gestation was associated with asthma development in boys by age 6 years (Leon Hsu et al. 2015). Animal studies show that perinatal exposure results in persistent airway hyperresponsiveness (Fanucchi et al. 2006; Manners et al. 2014; Mauad et al. 2008).
The biological effect of an air pollution particle is dependent on complexation of host iron by the particle with diminished metal availability (Fig. 4) (Ghio et al. 2015a). Polycyclic aromatic hydrocarbons, found in air pollution particles, and metabolized products can alter iron homeostasis by complexing cell iron (Schreinemachers and Ghio 2016). Components of air pollution other than particles (e.g. ozone) have similarly been associated with a disruption in iron homeostasis (Ghio et al. 2014; Ghio et al. 2007). These data support a relationship between asthma, exposure to air pollutants, and iron deficiency.
Particles other than those included in cigarette smoke and air pollution
Asthma can be associated with exposure to numerous, disparate particles. The weight of indoor dust samples taken in the home correlated with asthma (Elliott et al. 2007). Total suspended particles from a pre-harvest sugar cane burning had an acute effect on asthma admissions, starting at day one and remaining elevated for four days (Arbex et al. 2007). Likewise, exposures to particles other than cigarette smoking and air pollution particles are associated with decrements in pulmonary function and bronchial hyperreactivity (Ames et al. 1982; Lapp et al. 1972; Sundblad et al. 2002). Regarding transgenerational asthma, cooking with a wood stove during pregnancy, yet another particle-related exposure, is similarly associated with both asthma in children (as well as low birthweights) (Siddiqui et al. 2008).
All particle exposures are associated with a disruption in iron homeostasis (Ghio et al. 2015a, 2013). Surface functional groups will complex lung iron following inhalation and deposition (Fig. 4). While total iron will increase, the available metal will decrease. A functional deficiency in iron results, and this will impact the risk for asthma.
Occupational exposures (non-particulate)
There are numerous work-related exposures, which are associated with asthma. Comparable to other asthma phenotypes, individuals with several types of occupational asthma demonstrate a disruption in iron homeostasis and a functional deficiency of the metal. Inhalation of isocyanates is a leading cause of occupational asthma. These compounds have been demonstrated to disrupt iron homeostasis (Hur et al. 2008; Kim et al. 2010). Occupational exposure to various metals other than iron can also present as asthma or exacerbation of asthma (Daenen et al. 1999; Fernandez-Nieto et al. 2006; Irsigler et al. 1999; Malo et al. 1993; Sauni et al. 2010; Schneider et al. 2012; Wittczak et al. 2008). Exposure to metals other than iron has been shown to disrupt normal iron homeostasis in cells with a resulting functional deficiency (Ghio et al. 2015b). Exposure to cotton dust is associated with a loss in FEV1 across a work shift (i.e. byssinosis or “scheduled asthma”). Such decrements in pulmonary function with resulting symptoms of shortness of breath and wheezing have been observed to be more frequent among women who have diminished iron levels relative to males (Beck et al. 1982; Velazquez et al. 1991). Cotton is comprised of cellulose, a known iron chelator (Phillips and Fernandez 1981; Platt and Clydesdale 1984). Similar to particles, inhaled cotton dust is predicted to react with and accordingly diminish available iron in the respiratory tract. This effect has been observed as the formation of ferruginous bodies in the lung following cotton dust exposure (Spencer 1985).
Inhaled iron chelators
Direct exposure to compounds recognized to chelate iron can initiate respiratory symptoms and changes in pulmonary function consistent with asthma. Prominent among these compounds are citric acid and ethylenediaminetetraacetic acid (EDTA). Citric acid is diagnostically employed in cough provocation tests (Kastelik et al. 2002). Citric acid also causes increased bronchial responsiveness and bronchoconstriction (Ricciardolo et al. 1999). Among patients, there was an increase in airflow resistance reaching a maximum within 30 s after citric acid inhalation. This resistance returned to control values within one to three minutes (Simonsson et al. 1967). Exposure to EDTA, an iron chelator previously used as a preservative in inhalers, was considered responsible for an increase in airflow resistance associated with worsening of asthma (Brophy 1989). Complexation of airway iron by both citrate and EDTA is likely to result in a deficiency of iron leading to asthmatic symptoms and loss of pulmonary function.
Obesity
Obesity is a risk factor for asthma in all demographic groups; it is related more strongly to non-atopic (non-eosinophilic) than atopic airways disease (Raj et al. 2014). The pathophysiology underlying the association between obesity and asthma is poorly defined (Mohanan et al. 2014). Potential mechanisms of asthma in obese individuals could involve changes in the mechanical properties of the respiratory system, adipokine-mediated inflammation, chronic inflammation, gastro-esophageal reflux, and complications from sleep-disordered breathing (Ali and Ulrik 2013).
Iron homeostasis is disrupted by obesity. Obesity alters distribution of iron both at the cellular and tissue levels (Orr et al. 2014). Analysis employing public-use data files from the continuous NHANES (1999–2006) survey suggests a negative relationship between levels of blood iron content and individual body mass index after controlling for other individual characteristics (Neymotin and Sen 2011). This inverse correlation between serum iron and body mass index is observed despite elevations in serum iron with the same measure (Gillum 2001). Iron deficiency and anemia are frequent findings in subjects with progressed stages of obesity (Aigner et al. 2014; Nead et al. 2004). Obesity-associated inflammation is linked to disruptions in iron homeostasis (Cheng et al. 2012). In obese school-aged children, both iron status and inflammation are improved by weight reduction (Gong et al. 2014). The deficiency of iron can participate in the mechanistic pathway leading to asthma associated with obesity.
Maternal overweight increases the likelihood of childhood wheeze more than threefold (Kozyrskyj and Pawlowski 2013). This also correlates with a diminished availability of iron in the newborns of obese mothers and is likely a factor in transgenerational asthma (Phillips et al. 2014).
Anemias including hemoglobinopathies
The worldwide prevalence of anemia in 2010 was 32.9 % with over 2 billion people affected (i.e. almost one-third of the world was anemic) (Kassebaum et al. 2014). In a prospective-cohort study, anemia was found to be a risk factor for asthma among 200 children aged 2–18 years (Ramakrishnan and Borade 2010). Anemic children were 5.75 times more susceptible to asthma attacks when compared with nonanemic children, and iron deficiency was the most common cause (Lopez et al. 2015).
Sickle cell disease can commonly be associated with asthma (Anim et al. 2011; Miller and Gladwin 2012). The criteria for a physician diagnosis of asthma are not well defined in a patient with sickle cell disease. It is unclear whether sickle cell disease and asthma are distinct or overlapping co-morbid conditions (Field and DeBaun 2009). Accordingly, the true prevalence of co-existence is frequently underestimated. Significant overlap does exist in the clinical presentations of an episode of acute chest syndrome and asthma exacerbation. Supporting the concept that sickle cell disease and asthma can be the presentation of a singular process, there is evidence of a higher than expected prevalence of airway hyperreactivity and obstructive lung disease among patients with sickle cell disease without a diagnosis of asthma. Over 50 % of children with sickle cell disease show a positive response to methacholine while the prevalence of airway hyperreactivity is 18 % in children in the general population (Strunk et al. 2008).
Both iron deficiency anemia and iron deficiency have been documented in cohorts of patients with hemoglobinopathies including sickle cell anemia (Mohanty et al. 2008; Palma-Carlos et al. 2005). Sixty-seven percent of subjects with sickle cell anemia and 26 percent with sickle cell trait had evidence suggesting iron insufficiency and, following provision of iron, an improvement in hemoglobin levels can be observed in both sickle cell disorders. In addition, an acute phase reaction has been demonstrated to impact patients with sickle cell anemia (Singhal et al. 1993). Integral to the acute phase reaction, iron will be diminished reflecting a further decrease in availability of this metal. The lack of sufficient iron levels in hemoglobinopathies will increase the risk for asthma.
Autoimmune disease
The prevalence of bronchial hyperreactivity and asthma is increased in autoimmune diseases (Shen et al. 2014). While bronchial hyperreactivity was present in 6.5 % of the healthy controls, 25 % of the systemic sclerosis patients without associated Sjögren’s syndrome, 42.2 % of those with primary Sjögren’s syndrome, and 50 % of those with systemic sclerosis with associated Sjögren’s syndrome were reactive to methacholine (Gudbjornsson et al. 1991; La Corte et al. 1995; La Corte et al. 1992; Potena et al. 1990). Similarly, methacholine responsiveness was elevated among patients with rheumatoid arthritis (55 % arthritics vs. 16 % controls) (Hassan et al. 1994). Airflow obstruction is also significantly increased in these rheumatoid arthritis patients compared with controls.
A disruption in iron homeostasis has been found in autoimmune disease (Baynes et al. 1987; Blake et al. 1980; Palermo et al. 1986). Despite evidence of systemic iron deficiency, iron concentrations are elevated in involved synovium among patients diagnosed with rheumatoid arthritis (Blake et al. 1984; Giordano et al. 1991; Ogilvie-Harris and Fornaiser 1980). Iron deficiency and anemia are frequently associated with disease reflecting either an absolute or a functional insufficiency of the metal (Masson, 2011; van Steenbergen et al. 2013; Vanarsa et al. 2012). Intravenous iron has been employed in treating autoimmune disease with improvement of anemia and decreases in markers of inflammation (Duthie 1985). It is likely that this iron deficiency participates in respiratory disease related to autoimmune disease.
Surgery and critical illness
Surgical procedures can be complicated by asthma (Tirumalasetty and Grammer 2006). Iron deficiency develops post-surgically with serum concentrations and transferrin saturation reaching a nadir 48 h following the procedure (Solis et al. 2006). Other measured parameters reflect a disruption in iron homeostasis with decrements in red cell count, hemoglobin, and hematocrit. Transferrin-soluble receptors increase late in the post-operative course (days 30 and 45). There is not a complete recovery to normal iron homeostasis even after 45 days. Similarly, asthma and abnormalities in iron metabolism can be observed among patients with critical illness. Bronchial hyperreactivity is common among survivors of acute respiratory distress syndrome (Simpson et al. 1978). Those individuals diagnosed to have critical illness (e.g. acute respiratory distress syndrome) can demonstrate significant disruption of iron homeostasis along with an acute phase reaction, which negatively impacts iron availability (Ghio et al. 2003; Headley et al. 1997). The abnormal iron homeostasis after surgery and critical illness can contribute to asthma.
Medications
Certain medications are associated with increased asthma (Covar et al. 2005). The mechanistic pathway by which these medications trigger asthma is proposed to vary. Aspirin-exacerbated respiratory disease is characterized by hypersensitivity, asthma, and chronic rhinosinusitis with nasal polyps after use of aspirin and non-steroidal anti-inflammatory drugs. Aspirin-exacerbated respiratory disease affects 10–29 % asthmatic patients. Similarly, an asthma-like condition with cough develops in approximately 10 % of the patients treated with angiotensin converting enzyme (ACE) inhibitors, and the medication must be discontinued in approximately 50 % of these individuals (Overlack 1996). Beta-blockers can also be associated with bronchoconstriction and worsening of asthma (Short et al. 2012).
These medications, which affect asthma also impact iron import, accumulation, and release by cells (Komarov et al. 2006; Mak et al. 2006). Aspirin use is associated with diminished total iron stores reflected by lower serum ferritin concentrations (Fleming et al. 2001). ACE inhibitors decreased plasma iron concentration within 1–2 h after administration (Skrzypczak et al. 2010). Use of beta-blockers decreases iron availability (Komarov et al. 2006). These disruptions in iron homeostasis can lead to a deficiency of the metal thus increasing risk for asthma.
Stress
Psychosocial stress is linked to the expression of asthma (Cohen et al. 2008; Lange et al. 2011; Wright 2011). The poor are disproportionately affected by such stress and its associated asthma (Rosenberg et al. 2014). Psychosocial stress can similarly affect an acute phase response to diminish iron availability (de Rooij et al. 2007; Maes et al. 1996). The hypoferremia associated with the acute phase response is proposed to then impact asthma.
Co-morbidity of asthma
Co-morbid conditions of asthma can be associated with an absolute and a functional iron deficiency supporting a relationship of these diseases with a disruption in homeostasis of this metal. These co-morbid conditions can include eczema, urticaria, celiac disease, inflammatory bowel disease, migraine headaches, restless leg syndrome, and pulmonary hypertension.
Epidemiology has associated both eczema and urticaria with asthma (Ronchetti et al. 2002; Silverberg and Hanifin 2013). Comparable to wheeze consistent with asthma, umbilical cord blood concentrations of iron can inversely correlate with the later onset of eczema (Shaheen et al. 2004). Patients with atopic eczema have significantly lower levels of serum ferritin relative to controls (David et al. 1990). Low serum iron levels are also frequently observed among patients with chronic idiopathic urticaria while the restoration of normal serum iron is associated with either remission or clinical improvement (Guarneri et al. 2014; Wu et al. 2015).
The diagnosis of celiac disease increases the risk for asthma (Ludvigsson et al. 2011). Celiac disease is rare among individuals without iron deficiency (Karaman et al. 2016; Murray et al. 2013). Elevation of the total iron binding capacity is associated with an increased risk of celiac disease (Letner et al. 2015). Inflammatory bowel disease is a second gastrointestinal disease linked with asthma (Brassard et al. 2015). While not well defined, a disruption in iron homeostasis is obvious in inflammatory bowel disease with significant iron deficiency and anemias observed (Sukumaran et al. 2014; Wiskin et al. 2012).
Epidemiologic investigation supports migraine and asthma as co-morbid disorders (Chen and Leviton 1990; Davey et al. 2002; Wang et al. 2010). Patients with iron deficiency anemia have a high frequency of migraine headaches suggesting a relationship between disruption in the homeostasis of this metal and migraine headaches (Gur-Ozmen and Karahan-Ozcan 2015; Pamuk et al. 2015; Vukovic-Cvetkovic et al. 2010).
Current medication use among those with restless leg syndrome includes asthma/allergy drugs suggesting a possible association between the two (Pearson et al. 2008). Restless leg syndrome is a frequent sleep disorder that is linked to disturbed iron homeostasis. Among patients with iron deficiency anemia, the prevalence of clinically significant restless leg syndrome was 23.9 %, which is nine times higher than in the general population (Allen et al. 2013). Restless leg syndrome is more common during pregnancy (when iron deficiency is common) than in the general population, occurring at a 2–3 times higher prevalence, and intravenous iron can improve symptoms of restless leg syndrome during this time (Picchietti et al. 2012).
Finally, asthma and pulmonary arterial hypertension can be observed as co-morbidity. These two conditions share important pathological features including inflammation, smooth muscle contraction, and remodeling (Said et al. 2010). In both diseases there is increased resistance in airways and pulmonary arteries, respectively, due to airway and pulmonary vasoconstriction, smooth muscle constriction, and thickening of the walls caused by proliferation of smooth muscle and other cells (i.e. remodeling). Remodeling in asthma can include an increase in airway wall thickness, fibrosis, smooth muscle mass and vascularity, as well as abnormalities in extracellular matrix composition. Muscularization and remodeling of smaller pulmonary arteries are essential pathological lesions in pulmonary arterial hypertension. These shared pathological features between asthma and pulmonary arterial hypertension are unlikely to be strictly coincidental. Rather, they probably reflect a single underlying mechanism that is common to both diseases. The two diseases can also demonstrate overlapping clinical presentations (Achouh et al. 2008; Hayes 2007). Iron deficiency is common in patients with idiopathic pulmonary arterial hypertension and can also be associated with disease severity and poor clinical outcome (Decker et al. 2011; Rhodes et al. 2011). There can be a beneficial response among patients diagnosed with pulmonary arterial hypertension who are treated with oral iron (Ruiter et al. 2011). Iron availability modifies pulmonary arterial pressure and pulmonary vascular responses to hypoxia (Smith et al. 2008). In addition, hypoxic pulmonary hypertension may be attenuated by iron supplementation and exacerbated by iron depletion (Smith et al. 2009).
The relationship between these co-morbid conditions and disruptions of iron homeostasis support a role for diminished availability of the metal in asthma.
Treatment of asthma
Treatment for asthma can include pharmacologic agents, which impact iron homeostasis both directly and indirectly. Catecholamines regulate cellular iron homeostasis and deliver requisite iron to cells and organelles (Tapryal et al. 2015; Zhang et al. 2013). Inhaled therapies (corticosteroids alone or combined with beta-2 agonists) are a mainstay in asthma and reduce inflammation (Antoniu 2010; Tang et al. 2010). Through such a reduction in systemic inflammation, a normalization of iron availability is predicted.
The direct provision of iron has been demonstrated to impact animal models of asthma and measures of airway hyperreactivity in humans. Iron supplementation in a well-established mouse model of ovalbumin-driven allergic asthma resulted in a significant decrease in airway eosinophilia, while systemic iron injections led to a significant suppression of allergen-induced airway eosinophilia, Th2 cytokine release, and airway hyperreactivity compared to placebo (Maazi et al. 2011). Iron supplementation in iron-deficient women led to resolution of chronic cough and bronchial hyperreactivity further supporting an association of asthma and iron deficiency (Bucca et al. 2012).
Pathophysiologic events underlying asthma
Disruptions in iron homeostasis with decreased concentrations of available metal can initiate pathophysiologic events culminating in asthma. Such events include (1) inflammation and (2) muscle contraction involved in bronchoconstriction and obstruction.
Regarding the participation of iron in inflammation, changes in the availability of this metal activate numerous pathways that coordinate inflammation (Nakagawa et al. 2014). Cellular iron deficiency and its associated oxidative stress affect an activation of specific kinases including p38, JNK, ERK1, and ERK2 (Choi et al. 2007; Fan et al. 2011; Huang et al. 2007; Yu and Richardson 2011). Increasing the cell concentration of available iron (Ghio et al. 2013) can diminish phosphorylation of kinases following exposure to inflammatory agents. Comparable to kinase phosphorylation, diminished cell iron concentrations correspond to an activation of pro-inflammatory transcription factors including NF-κB, AP-1, HIF-α, and CREB (Fan et al. 2011; Lee et al. 2008; Lu et al. 2009; Varesio et al. 2010; Yuan et al. 2011). Inflammatory mediator release also increases with diminished availability of iron (Choi et al. 2007; Jeong et al. 2005; Lee et al. 2008; Varesio et al. 2010). The biological effects after exposure to inflammatory agents have been demonstrated to be related to iron homeostasis (Driscoll et al. 2001; Ghiazza et al. 2011).
Iron levels specifically affect the balance between and the intensity of Th1 and Th2 arms of the immune response with availability of the metal impacting the Th2 response (Gordeuk et al. 2009). Diminished iron availability during the development of the fetal immune system may result in a pattern of persistent Th2 dominance (Weigert et al. 2015). Th2 cells participate with M2 cells in reparative reactions including asthma (Martinez et al. 2009). The differentiation of these inflammatory cells can reflect iron homeostasis (Corna et al. 2010). Eosinophilia is an endpoint commonly employed as an index of risk for asthma. Iron deficiency can also correspond with eosinophilia (Weigert et al. 2015). Finally, decreased iron availability can lead to elevation of blood levels of IL-4 which participates in the Th2 response (Naderi et al. 2013; Nyakeriga et al. 2005).
Muscle contraction demonstrates a relationship with iron concentration. Negative chronotropic and inotropic effects were observed with inclusion of iron in monolayer cultures of ventricular myocytes (Moreb et al. 1988). Iron reduces peak-developed tension and the maximal rate of tension development in isometrically contracting left atrial strips and right ventricular papillary muscles (Artman et al. 1984a). Iron depresses myocardial contractility as evidenced by dose-related declines in contractile function in isolated isometrically contracting rabbit left atrial strips and right ventricular papillary muscles (Artman et al. 1982, 1984a, 1984b). In contrast, anemic infants with decreased iron concentrations showed increases in cardiac contractility, stroke volume, and left ventricular output period (Cambonie et al. 2007). Such enhanced contractility (left ventricular performance) in anemia can also be observed among adults (Florenzano et al. 1984). Changes in muscle contractility associated with anemia reverse with blood transfusion (Cambonie et al. 2007). In skeletal muscle, iron exposure is associated with diminished force in a strength test (Reardon and Allen 2009). Finally, elevated iron availability impacts venous dilation, possibly through a negative ionotropic effect (Reissman et al. 1955; Robotham and Lietman 1980; Whitten and Brough 1971, 1968).
Conclusions
A relationship between iron deficiency and asthma is supported by direct measures of the metal, evaluation of determinants (demographic, physiologic, and pathologic) of the disease, analysis of co-morbid conditions, comprehension of therapies, and analysis of the pathophysiological events. The determinants are predicted both to interact in lowering iron and to demonstrate synergy in impacting asthma (e.g. exercise and gender both influence iron homeostasis and are anticipated to contribute to an even greater risk for asthma among female athletes) (Fallon 2004; Landahl et al. 2005; Ostojic and Ahmetovic 2008; Reinke et al. 2012). A recognition of the association between iron deficiency and asthma suggests preventive and therapeutic interventions. The prevention of iron deficiency may necessitate preclusion of exposure to specific compounds, which can either function as or be metabolized to inappropriate iron chelators. Several of these compounds associated with disruption of iron homeostasis and asthma have found use in restricting the growth of microbes and plants (e.g. pesticides, antibiotics, and preservatives). Unfortunately, it appears that these same agents can impact the human in an identical manner to disrupt iron homeostasis, produce a metal deficiency, and initiate disease. While exclusion of such compounds from the environment of the lower respiratory tract can be recommended, systemic prohibition might be required. It is unclear if elimination of such exposures is possible.
The relationship between iron deficiency and asthma reveals a potential for treatment with iron. Treatment with oral supplementation can be tested. In addition, intravenous iron might be of benefit especially since it has been employed in the treatment of other diseases in which deficiency exists (e.g. restless leg syndrome) (Lieske et al. 2015). In these disease states, a threshold effect of ferritin (i.e. iron storage) has been suggested. In restless leg syndrome, iron supplementation to achieve ferritin levels of at least 50 ng/ml has been proposed (Kantor et al. 2003; Leschziner and Gringras 2012; Trost et al. 2006). It should be noted that intravenous iron is not always successful in reversing a deficiency (Hogan et al. 2015; Liles 2012). With iron deficiency associated with an acute phase response, intravenous replacement frequently fails (El-Khatib et al. 2006). However, inhaled iron presents a therapeutic approach to treating asthma as an iron deficiency unique to the lung. The inhaled iron could be presented as salts (e.g. iron sulfate) or nanoparticles (e.g. iron oxide), which can be tolerated by humans (Kleinman et al. 1981). While it is expected that some forms of asthma (e.g. asthma associated with active infections) will not be amenable to such an approach addition of ancillary iron has the potential to provide a new treatment for asthma.
References
Achouh L et al (2008) Pulmonary arterial hypertension masquerading as severe refractory asthma. Eur Respir J 32:513–516. doi:10.1183/09031936.00005408
Adriaanse HP, Knottnerus JA, Delgado LR, Cox HH, Essed GG (1996) Smoking in Dutch pregnant women and birth weight. Patient Educ Couns 28:25–30
Aigner E, Feldman A, Datz C (2014) Obesity as an emerging risk factor for iron deficiency. Nutrients 6:3587–3600. doi:10.3390/nu6093587
Ali Z, Ulrik CS (2013) Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med 107:1287–1300. doi:10.1016/j.rmed.2013.03.019
Allen LH (2001) Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J Nutr 131:581S–589S
Allen RP, Auerbach S, Bahrain H, Auerbach M, Earley CJ (2013) The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol 88:261–264. doi:10.1002/ajh.23397
Almqvist C, Worm M, Leynaert B, Working Group of GALENWPG (2008) Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 63:47–57. doi:10.1111/j.1398-9995.2007.01524.x
American College of Obstetricians and Gynecologists (2008) ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 112:201–207. doi:10.1097/AOG.0b013e3181809c0d
Ames RG, Attfield MD, Hankinson JL, Hearl FJ, Reger RB (1982) Acute respiratory effects of exposure to diesel emissions in coal miners. Am Rev Respir Dis 125:39–42
Andersen SA, Petersen HH, Ersboll AK, Falk-Ronne J, Jacobsen S (2012) Vaccination elicits a prominent acute phase response in horses. Vet J 191:199–202. doi:10.1016/j.tvjl.2011.01.019
Anderson SD (2012) The prevention of exercise-induced bronchoconstriction: what are the options? Expert Rev Respir Med 6:355–357. doi:10.1586/ers.12.33
Anim SO, Strunk RC, DeBaun MR (2011) Asthma morbidity and treatment in children with sickle cell disease. Expert Rev Respir Med 5:635–645. doi:10.1586/ers.11.64
Antoniu SA (2010) Effects of inhaled therapy on biomarkers of systemic inflammation in stable chronic obstructive pulmonary disease. Biomarkers 15:97–103. doi:10.3109/13547500903311902
Aranda N, Ribot B, Garcia E, Viteri FE, Arija V (2011) Pre-pregnancy iron reserves, iron supplementation during pregnancy, and birth weight. Early Hum Dev 87:791–797. doi:10.1016/j.earlhumdev.2011.06.003
Arbex MA et al (2007) Air pollution from biomass burning and asthma hospital admissions in a sugar cane plantation area in Brazil. J Epidemiol Community Health 61:395–400. doi:10.1136/jech.2005.044743
Artman M, Olson RD, Boerth RC (1982) Depression of myocardial contractility in acute iron toxicity in rabbits. Toxicol Appl Pharmacol 66:329–337
Artman M, Olson RD, Boucek RJ Jr, Boerth RC (1984a) Depression of contractility in isolated rabbit myocardium following exposure to iron: role of free radicals. Toxicol Appl Pharmacol 72:324–332
Artman M, Olson RD, Boucek RJ Jr, Ghishan FK, Boerth RC (1984b) Acute effects of iron on contractile function in isolated rabbit myocardium. Dev Pharmacol Ther 7:50–60
Auersperger I, Skof B, Leskosek B, Knap B, Jerin A, Lainscak M (2013) Exercise-induced changes in iron status and hepcidin response in female runners. PLoS One 8:e58090. doi:10.1371/journal.pone.0058090
Badal S, Her YF, Maher LJ 3rd (2015) Nonantibiotic effects of fluoroquinolones in mammalian cells. J Biol Chem 290:22287–22297. doi:10.1074/jbc.M115.671222
Baker RD, Greer FR, Committee on Nutrition American Academy of P (2010) Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 126:1040–1050. doi:10.1542/peds.2010-2576
Barker DJ, Osmond C, Forsen TJ, Thornburg KL, Kajantie E, Eriksson JG (2013) Foetal and childhood growth and asthma in adult life. Acta Paediatr 102:732–738. doi:10.1111/apa.12257
Baynes RD, Bothwell TH, Bezwoda WR, Gear AJ, Atkinson P (1987) Hematologic and iron-related measurements in rheumatoid arthritis. Am J Clin Pathol 87:196–200
Beaudoin S, Tremblay GM, Croitoru D, Benedetti A, Landry JS (2013) Healthcare utilization and health-related quality of life of adult survivors of preterm birth complicated by bronchopulmonary dysplasia. Acta Paediatr 102:607–612. doi:10.1111/apa.12217
Beck GJ, Schachter EN, Maunder LR, Schilling RS (1982) A prospective study of chronic lung disease in cotton textile workers. Ann Intern Med 97:645–651
Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A (2014) Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med 11:e1001596. doi:10.1371/journal.pmed.1001596
Beguin Y (1992) The soluble transferrin receptor: biological aspects and clinical usefulness as quantitative measure of erythropoiesis. Haematologica 77:1–10
Benn CS et al (2002) Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood. J Allergy Clin Immunol 110:72–77
Bhargava M, Iyer PU, Kumar R, Ramji S, Kapani V, Bhargava SK (1991) Relationship of maternal serum ferritin with foetal serum ferritin, birth weight and gestation. J Trop Pediatr 37:149–152
Bjerg A, Hedman L, Perzanowski M, Lundback B, Ronmark E (2011) A strong synergism of low birth weight and prenatal smoking on asthma in schoolchildren. Pediatrics 127:e905–912. doi:10.1542/peds.2010-2850
Blake DR, Scott DG, Eastham EJ, Rashid H (1980) Assessment of iron deficiency in rheumatoid arthritis. Br Med J 280:527
Blake DR, Gallagher PJ, Potter AR, Bell MJ, Bacon PA (1984) The effect of synovial iron on the progression of rheumatoid disease. A histologic assessment of patients with early rheumatoid synovitis. Arthritis Rheum 27:495–501
Bothwell TH (2000) Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 72:257S–264S
Boulet LP, O’Byrne PM (2015) Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med 372:641–648. doi:10.1056/NEJMra1407552
Brassard P, Vutcovici M, Ernst P, Patenaude V, Sewitch M, Suissa S, Bitton A (2015) Increased incidence of inflammatory bowel disease in Quebec residents with airway diseases. Eur Respir J 45:962–968. doi:10.1183/09031936.00079414
Brauer M et al (2002) Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med 166:1092–1098. doi:10.1164/rccm.200108-007OC
Brigham EP, McCormack MC, Takemoto CM, Matsui EC (2015) Iron status is associated with asthma and lung function in US women. PLoS One 10:e0117545. doi:10.1371/journal.pone.0117545
Brophy CJ (1989) The drug treatment of asthma. Postgrad Med J 65:506–507
Brosnahan MM, Erb HN, Perkins GA, Divers TJ, Borges AS, Osterrieder N (2012) Serum iron parameters and acute experimental EHV-1 infection in horses. J Vet Intern Med 26:1232–1235. doi:10.1111/j.1939-1676.2012.00963.x
Brostrom EB, Akre O, Katz-Salamon M, Jaraj D, Kaijser M (2013) Obstructive pulmonary disease in old age among individuals born preterm. Eur J Epidemiol 28:79–85. doi:10.1007/s10654-013-9761-7
Bruce FC et al (2008) Maternal morbidity rates in a managed care population. Obstet Gynecol 111:1089–1095. doi:10.1097/AOG.0b013e31816c441a
Bucca C et al (2012) Effect of iron supplementation in women with chronic cough and iron deficiency. Int J Clin Pract 66:1095–1100. doi:10.1111/ijcp.12001
Burke H et al (2012) Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 129:735–744. doi:10.1542/peds.2011-2196
Burr ML, Wat D, Evans C, Dunstan FD, Doull IJ, British Thoracic Society Research C (2006) Asthma prevalence in 1973, 1988 and 2003. Thorax 61:296–299. doi:10.1136/thx.2005.045682
Burrows B, Sears MR, Flannery EM, Herbison GP, Holdaway MD, Silva PA (1995) Relation of the course of bronchial responsiveness from age 9 to age 15 to allergy. Am J Respir Crit Care Med 152:1302–1308. doi:10.1164/ajrccm.152.4.7551386
Busse WW, Lemanske RF Jr, Gern JE (2010) Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 376:826–834. doi:10.1016/S0140-6736(10)61380-3
Butensky James E, Harmatz P, Lee M, Kennedy C, Petru A, Wara D, Miaskowski C (2009) Altered iron metabolism in children with human immunodeficiency virus disease. Pediatr Hematol Oncol 26:69–84. doi:10.1080/08880010902754826
Cambonie G, Matecki S, Milesi C, Voisin M, Guillaumont S, Picaud JC (2007) Myocardial adaptation to anemia and red blood cell transfusion in premature infants requiring ventilation support in the 1st postnatal week. Neonatology 92:174–181. doi:10.1159/000101568
Campo P et al (2006) Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol 118:1271–1278. doi:10.1016/j.jaci.2006.08.008
Cao GY, Li Y, Jin PF, Hu X (2012) Circadian rhythm in serum iron levels. Biol Trace Elem Res 147:63–66. doi:10.1007/s12011-011-9304-6
Celedon JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TA, Gold DR (2007) Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol 120:144–149. doi:10.1016/j.jaci.2007.03.037
Cemeroglu AP, Ozsoylu S (1994) Haematologic consequences of viral infections including serum iron status. Eur J Pediatr 153:171–173
Cetin I, Berti C, Mando C, Parisi F (2011) Placental iron transport and maternal absorption. Ann Nutr Metab 59:55–58. doi:10.1159/000332133
Chambers EC, Heshka S, Gallagher D, Wang J, Pi-Sunyer FX, Pierson RN Jr (2006) Serum iron and body fat distribution in a multiethnic cohort of adults living in New York City. J Am Diet Assoc 106:680–684. doi:10.1016/j.jada.2006.02.013
Chelchowska M, Laskowska-Klita T (2002) Effect of maternal smoking on some markers of iron status in umbilical cord blood. Roczniki Akademii Medycznej w Bialymstoku 47:235–240
Chen TC, Leviton A (1990) Asthma and eczema in children born to women with migraine. Arch Neurol 47:1227–1230
Cheng HL, Bryant C, Cook R, O’Connor H, Rooney K, Steinbeck K (2012) The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev 13:150–161. doi:10.1111/j.1467-789X.2011.00938.x
Cheung D, Dick EC, Timmers MC, de Klerk EP, Spaan WJ, Sterk PJ (1995) Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med 152:1490–1496. doi:10.1164/ajrccm.152.5.7582282
Choi EY et al (2007) Transcriptional regulation of IL-8 by iron chelator in human epithelial cells is independent from NF-kappaB but involves ERK1/2- and p38 kinase-dependent activation of AP-1. J Cell Biochem 102:1442–1457. doi:10.1002/jcb.21367
Cohen RT, Canino GJ, Bird HR, Celedon JC (2008) Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med 178:453–459. doi:10.1164/rccm.200711-1629OC
Collard KJ (2009) Iron homeostasis in the neonate. Pediatrics 123:1208–1216. doi:10.1542/peds.2008-1047
Corna G et al (2010) Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 95:1814–1822. doi:10.3324/haematol.2010.023879
Covar RA, Macomber BA, Szefler SJ (2005) Medications as asthma triggers. Immunol Allergy Clin North Am 25:169–190. doi:10.1016/j.iac.2004.09.009
Cox B, Martens E, Nemery B, Vangronsveld J, Nawrot TS (2013) Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. BMJ 346:f441. doi:10.1136/bmj.f441
Dadvand P et al (2013) Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect 121:267–373. doi:10.1289/ehp.1205575
Daenen M, Rogiers P, Van de Walle C, Rochette F, Demedts M, Nemery B (1999) Occupational asthma caused by palladium. Eur Respir J 13:213–216
Dallman PR, Siimes MA, Manies EC (1975) Brain iron: persistent deficiency following short-term iron deprivation in the young rat. Br J Haematol 31:209–215
Dally MB, Kurrle S, Breslin AB (1978) Ventilatory effects of aerosol gentamicin. Thorax 33:54–56
Damgaard AL, Hansen BM, Mathiasen R, Buchvald F, Lange T, Greisen G (2015) Prematurity and prescription asthma medication from childhood to young adulthood: a Danish national cohort study. PLoS One 10:e0117253. doi:10.1371/journal.pone.0117253
Davey G, Sedgwick P, Maier W, Visick G, Strachan DP, Anderson HR (2002) Association between migraine and asthma: matched case-control study. Br J Gen Pract 52:723–727
David TJ, Wells FE, Sharpe TC, Gibbs AC, Devlin J (1990) Serum levels of trace metals in children with atopic eczema. Br J Dermatol 122:485–489
de Rooij SE, van Munster BC, Korevaar JC, Levi M (2007) Cytokines and acute phase response in delirium. J Psychosom Res 62:521–525. doi:10.1016/j.jpsychores.2006.11.013
Debley JS, Redding GJ, Critchlow CW (2004) Impact of adolescence and gender on asthma hospitalization: a population-based birth cohort study. Pediatr Pulmonol 38:443–450. doi:10.1002/ppul.20108
Decker I et al (2011) High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci 4:253–258. doi:10.1111/j.1752-8062.2011.00301.x
Demissie K, Breckenridge MB, Rhoads GG (1998) Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med 158:1091–1095. doi:10.1164/ajrccm.158.4.9802053
Dezateux C, Stocks J, Dundas I, Fletcher ME (1999) Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med 159:403–410. doi:10.1164/ajrccm.159.2.9712029
Di Santolo M, Stel G, Banfi G, Gonano F, Cauci S (2008) Anemia and iron status in young fertile non-professional female athletes. Eur J Appl Physiol 102:703–709. doi:10.1007/s00421-007-0647-9
Dodge RR, Burrows B (1980) The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis 122:567–575. doi:10.1164/arrd.1980.122.4.567
Drake KA, Galanter JM, Burchard EG (2008) Race, ethnicity and social class and the complex etiologies of asthma. Pharmacogenomics 9:453–462. doi:10.2217/14622416.9.4.453
Drakesmith H, Prentice A (2008) Viral infection and iron metabolism. Nat Rev Microbiol 6:541–552. doi:10.1038/nrmicro1930
Dratva J et al (2010) Perimenstrual increase in bronchial hyperreactivity in premenopausal women: results from the population-based SAPALDIA 2 cohort. J Allergy Clin Immunol 125:823–829. doi:10.1016/j.jaci.2009.12.938
Driscoll KE, Howard BW, Carter JM, Janssen YM, Mossman BT, Isfort RJ (2001) Mitochondrial-derived oxidants and quartz activation of chemokine gene expression. Adv Exp Med Biol 500:489–496
Duthie JJ (1985) Intravenous iron in the treatment of rheumatoid arthritis. Ann Rheum Dis 44:572–573
Duvigneau JC et al (2008) A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest 88:70–77. doi:10.1038/labinvest.3700691
Ekmekci OB, Donma O, Sardogan E, Yildirim N, Uysal O, Demirel H, Demir T (2004) Iron, nitric oxide, and myeloperoxidase in asthmatic patients. Biochemistry (Mosc) 69:462–467
Eliasson O, Scherzer HH, DeGraff AC Jr (1986) Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol 77:87–94
El-Khatib M, Duncan HJ, Kant KS (2006) Role of C-reactive protein, reticulocyte haemoglobin content and inflammatory markers in iron and erythropoietin administration in dialysis patients. Nephrology (Carlton) 11:400–404. doi:10.1111/j.1440-1797.2006.00676.x
Elliott L et al (2007) Dust weight and asthma prevalence in the National Survey of Lead and Allergens in Housing (NSLAH). Environ Health Perspect 115:215–220. doi:10.1289/ehp.9412
Emond AM, Hawkins N, Pennock C, Golding J (1996) Haemoglobin and ferritin concentrations in infants at 8 months of age. Arch Dis Child 74:36–39
Fallon KE (2004) Utility of hematological and iron-related screening in elite athletes. Clin J Sport Med 14:145–152
Fan Y, Wang J, Wei L, He B, Wang C, Wang B (2011) Iron deficiency activates pro-inflammatory signaling in macrophages and foam cells via the p38 MAPK-NF-kappaB pathway. Int J Cardiol 152:49–55. doi:10.1016/j.ijcard.2010.07.005
Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES (2006) Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol 291:L644–650. doi:10.1152/ajplung.00027.2006
Farha S, Asosingh K, Laskowski D, Hammel J, Dweik RA, Wiedemann HP, Erzurum SC (2009) Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med 180:304–310. doi:10.1164/rccm.200904-0497OC
Fernandez-Nieto M, Quirce S, Carnes J, Sastre J (2006) Occupational asthma due to chromium and nickel salts. Int Arch Occup Environ Health 79:483–486. doi:10.1007/s00420-005-0078-z
Ferrandiz MJ, de la Campa AG (2014) The fluoroquinolone levofloxacin triggers the transcriptional activation of iron transport genes that contribute to cell death in Streptococcus pneumoniae. Antimicrob Agents Chemother 58:247–257. doi:10.1128/AAC.01706-13
Ferri C, Procianoy RS, Silveira RC (2014) Prevalence and risk factors for iron-deficiency anemia in very-low-birth-weight preterm infants at 1 year of corrected age. J Trop Pediatr 60:53–60. doi:10.1093/tropej/fmt077
Field JJ, DeBaun MR (2009) Asthma and sickle cell disease: two distinct diseases or part of the same process? Hematol Am Soc Hematol Educ Program 2009:45–53. doi:10.1182/asheducation-2009.1.45
Fillebeen C, Pantopoulos K (2013) Hepatitis C virus infection causes iron deficiency in Huh7.5.1 cells. PLoS One 8:e83307. doi:10.1371/journal.pone.0083307
Fiori A, Van Dijck P (2012) Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob Agents Chemother 56:3785–3796. doi:10.1128/AAC.06017-11
Fleming DJ, Jacques PF, Massaro JM, D’Agostino RB Sr, Wilson PW, Wood RJ (2001) Aspirin intake and the use of serum ferritin as a measure of iron status. Am J Clin Nutr 74:219–226
Florenzano F, Diaz G, Regonesi C, Escobar E (1984) Left ventricular function in chronic anemia: evidence of noncatecholamine positive inotropic factor in the serum. Am J Cardiol 54:638–645
Friedman MS, Powell KE, Hutwagner L, Graham LM, Teague WG (2001) Impact of changes in transportation and commuting behaviors during the Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA 285:897–905
Gauthier M, Ray A, Wenzel SE (2015) Evolving concepts of asthma. Am J Respir Crit Care Med 192:660–668. doi:10.1164/rccm.201504-0763PP
Gent JF, Koutrakis P, Belanger K, Triche E, Holford TR, Bracken MB, Leaderer BP (2009) Symptoms and medication use in children with asthma and traffic-related sources of fine particle pollution. Environ Health Perspect 117:1168–1174. doi:10.1289/ehp.0800335
Ghiazza M et al (2011) Surface iron inhibits quartz-induced cytotoxic and inflammatory responses in alveolar macrophages. Chem Res Toxicol 24:99–110. doi:10.1021/tx1003003
Ghio AJ, Carter JD, Richards JH, Richer LD, Grissom CK, Elstad MR (2003) Iron and iron-related proteins in the lower respiratory tract of patients with acute respiratory distress syndrome. Crit Care Med 31:395–400. doi:10.1097/01.CCM.0000050284.35609.97
Ghio AJ et al (2007) Lung injury after ozone exposure is iron dependent. Am J Physiol Lung Cell Mol Physiol 292:L134–143. doi:10.1152/ajplung.00534.2005
Ghio AJ et al (2013) Sequestration of mitochondrial iron by silica particle initiates a biological effect. Am J Physiol Lung Cell Mol Physiol 305:L712–724. doi:10.1152/ajplung.00099.2013
Ghio AJ, Soukup JM, Dailey LA, Richards JH, Duncan KE, Lehmann J (2014) Iron decreases biological effects of ozone exposure. Inhal Toxicol 26:391–399. doi:10.3109/08958378.2014.908330
Ghio AJ, Soukup JM, Dailey LA, Tong H, Kesic MJ, Budinger GR, Mutlu GM (2015a) Wood smoke particle sequesters cell iron to impact a biological effect. Chem Res Toxicol 28:2104–2111. doi:10.1021/acs.chemrestox.5b00270
Ghio AJ, Stonehuerner J, Soukup JM, Dailey LA, Kesic MJ, Cohen MD (2015b) Iron diminishes the in vitro biological effect of vanadium. J Inorg Biochem 147:126–133. doi:10.1016/j.jinorgbio.2015.03.008
Gillum RF (2001) Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 25:639–645. doi:10.1038/sj.ijo.0801561
Ginsburg GS, Agil A, O’Toole M, Rimm E, Douglas PS, Rifai N (1996) Effects of a single bout of ultraendurance exercise on lipid levels and susceptibility of lipids to peroxidation in triathletes. JAMA 276:221–225
Giordano N et al (1991) Histopathological study of iron deposit distribution in the rheumatoid synovium. Clin Exp Rheumatol 9:463–467
Godfrey S, Bar-Yishay E (1993) Exercised-induced asthma revisited. Respir Med 87:331–344
Gong L et al (2014) Weight loss, inflammatory markers, and improvements of iron status in overweight and obese children. J Pediatr 164(795–800):e792. doi:10.1016/j.jpeds.2013.12.004
Gordeuk VR et al (2009) Circulating cytokines in pulmonary tuberculosis according to HIV status and dietary iron content. Int J Tuberc Lung Dis 13:1267–1273
Gorten MK, Cross ER (1964) Iron metabolism in premature infants. II Prevention of iron deficiency. J Pediatr 64:509–520
Gregory A, Doull I, Pearce N, Cheng S, Leadbitter P, Holgate S, Beasley R (1999) The relationship between anthropometric measurements at birth: asthma and atopy in childhood. Clin Exp Allergy 29:330–333
Guarneri F, Guarneri C, Cannavo SP (2014) Oral iron therapy and chronic idiopathic urticaria: sideropenic urticaria? Dermatol Ther 27:223–226. doi:10.1111/dth.12122
Guarnieri M, Balmes JR (2014) Outdoor air pollution and asthma. Lancet 383:1581–1592. doi:10.1016/S0140-6736(14)60617-6
Gudbjornsson B, Hedenstrom H, Stalenheim G, Hallgren R (1991) Bronchial hyperresponsiveness to methacholine in patients with primary Sjogren’s syndrome. Ann Rheum Dis 50:36–40
Guiang SF 3rd, Georgieff MK, Lambert DJ, Schmidt RL, Widness JA (1997) Intravenous iron supplementation effect on tissue iron and hemoproteins in chronically phlebotomized lambs. Am J Physiol 273:R2124–2131
Guilbert TW, Denlinger LC (2010) Role of infection in the development and exacerbation of asthma. Expert Rev Respir Med 4:71–83. doi:10.1586/ers.09.60
Gunnarsson BS, Thorsdottir I, Palsson G (2004) Iron status in 2-year-old Icelandic children and associations with dietary intake and growth. Eur J Clin Nutr 58:901–906. doi:10.1038/sj.ejcn.1601910
Gur-Ozmen S, Karahan-Ozcan R (2015) Iron deficiency anemia is associated with menstrual migraine: a case-control study. Pain Med. doi:10.1093/pm/pnv029
Haldar P et al (2008) Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 178:218–224. doi:10.1164/rccm.200711-1754OC
Hassan WU, Henderson AF, Keaney NP (1992) Influenza vaccination in asthma. Lancet 339:194
Hassan WU, Keaney NP, Holland CD, Kelly CA (1994) Bronchial reactivity and airflow obstruction in rheumatoid arthritis. Ann Rheum Dis 53:511–514
Hayes D Jr (2007) Idiopathic pulmonary arterial hypertension misdiagnosed as asthma. J Asthma 44:19–22. doi:10.1080/02770900601125243
He H et al (2015) Preterm birth with childhood asthma: the role of degree of prematurity and asthma definitions. Am J Respir Crit Care Med 192:520–523. doi:10.1164/rccm.201503-0522LE
Headley AS, Tolley E, Meduri GU (1997) Infections and the inflammatory response in acute respiratory distress syndrome. Chest 111:1306–1321
Heegaard PM, Godson DL, Toussaint MJ, Tjornehoj K, Larsen LE, Viuff B, Ronsholt L (2000) The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet Immunol Immunopathol 77:151–159
Heinrich J, Wichmann HE (2004) Traffic related pollutants in Europe and their effect on allergic disease. Curr Opin Allergy Clin Immunol 4:341–348
Helenius IJ, Tikkanen HO, Haahtela T (1997) Association between type of training and risk of asthma in elite athletes. Thorax 52:157–160
Helenius IJ, Tikkanen HO, Sarna S, Haahtela T (1998) Asthma and increased bronchial responsiveness in elite athletes: atopy and sport event as risk factors. J Allergy Clin Immunol 101:646–652. doi:10.1016/S0091-6749(98)70173-3
Hemminki E, Rimpela U (1991) A randomized comparison of routine versus selective iron supplementation during pregnancy. J Am Coll Nutr 10:3–10
Hemminki E, Starfield B (1978) Routine administration of iron and vitamins during pregnancy: review of controlled clinical trials. Br J Obstet Gynaecol 85:404–410
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297
Hileti D, Panayiotidis P, Hoffbrand AV (1995) Iron chelators induce apoptosis in proliferating cells. Br J Haematol 89:181–187
Hogan M, Klein AA, Richards T (2015) The impact of anaemia and intravenous iron replacement therapy on outcomes in cardiac surgery. Eur J Cardiothorac Surg 47:218–226. doi:10.1093/ejcts/ezu200
Hokama T (1999) Relationship between maternal and cord blood plasma iron concentrations. J Trop Pediatr 45:120
Hollams EM, de Klerk NH, Holt PG, Sly PD (2014) Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med 189:401–407. doi:10.1164/rccm.201302-0323OC
Hoppe M, Hulthen L (2007) Capturing the onset of the common cold and its effects on iron absorption. Eur J Clin Nutr 61:1032–1034. doi:10.1038/sj.ejcn.1602614
Huang X, Dai J, Huang C, Zhang Q, Bhanot O, Pelle E (2007) Deferoxamine synergistically enhances iron-mediated AP-1 activation: a showcase of the interplay between extracellular-signal-regulated kinase and tyrosine phosphatase. Free Radic Res 41:1135–1142. doi:10.1080/10715760701609061
Hur GY et al (2008) Serum ferritin and transferrin levels as serologic markers of methylene diphenyl diisocyanate-induced occupational asthma. J Allergy Clin Immunol 122:774–780. doi:10.1016/j.jaci.2008.07.034
Irsigler GB, Visser PJ, Spangenberg PA (1999) Asthma and chemical bronchitis in vanadium plant workers. Am J Ind Med 35:366–374
Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE (2015) understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med 192:134–156. doi:10.1164/rccm.201412-2142PP
James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, Musk AW (2005) Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med 171:109–114. doi:10.1164/rccm.200402-230OC
Jedrychowski W, Galas A, Whyatt R, Perera F (2006) The prenatal use of antibiotics and the development of allergic disease in one year old infants. A preliminary study. Int J Occup Med Environ Health 19:70–76
Jeon YH, Yang HJ, Pyun BY (2009) Lung function in Korean adolescent girls: in association with obesity and the menstrual cycle. J Korean Med Sci 24:20–25. doi:10.3346/jkms.2009.24.1.20
Jeong HJ, Hong SH, Park RK, Shin T, An NH, Kim HM (2005) Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1, and NF-kappaB on HEI-OC1 cells. Hear Res 207:59–67. doi:10.1016/j.heares.2005.04.003
Kaneshige E (1981) Serum ferritin as an assessment of iron stores and other hematologic parameters during pregnancy. Obstet Gynecol 57:238–242
Kantor J, Kessler LJ, Brooks DG, Cotsarelis G (2003) Decreased serum ferritin is associated with alopecia in women. J Investig Dermatol 121:985–988. doi:10.1046/j.1523-1747.2003.12540.x
Karaman K, Akbayram S, Kar S, Demiroren K (2016) Prevalence of celiac disease in children with iron deficiency anemia in Van lake region of Turkey. J Pediatr Hematol Oncol 38:143–146. doi:10.1097/MPH.0000000000000495
Karimi P, Peters KO, Bidad K, Strickland PT (2015) Polycyclic aromatic hydrocarbons and childhood asthma. Eur J Epidemiol 30:91–101. doi:10.1007/s10654-015-9988-6
Kassebaum NJ et al (2014) A systematic analysis of global anemia burden from 1990 to 2010. Blood 123:615–624. doi:10.1182/blood-2013-06-508325
Kastelik JA, Thompson RH, Aziz I, Ojoo JC, Redington AE, Morice AH (2002) Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med 166:961–964. doi:10.1164/rccm.2109061
Kelly AM, MacDonald DJ, McDougall AN (1978) Observations on maternal and fetal ferritin concentrations at term. Br J Obstet Gynaecol 85:338–343
Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D (2005) Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 106:1864–1866. doi:10.1182/blood-2005-03-1159
Kennedy MC, Mende-Mueller L, Blondin GA, Beinert H (1992) Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci USA 89:11730–11734
Kim I, Yetley EA, Calvo MS (1993) Variations in iron-status measures during the menstrual cycle. Am J Clin Nutr 58:705–709
Kim SH, Choi GS, Ye YM, Jou I, Park HS, Park SM (2010) Toluene diisocyanate (TDI) regulates haem oxygenase-1/ferritin expression: implications for toluene diisocyanate-induced asthma. Clin Exp Immunol 160:489–497. doi:10.1111/j.1365-2249.2010.04118.x
Kim SE et al (2013) Diagnostic use of serum ferritin levels to differentiate infectious and noninfectious diseases in patients with fever of unknown origin. Dis Markers 34:211–218. doi:10.3233/DMA-130962
Kleinman MT, Linn WS, Bailey RM, Anderson KR, Whynot JD, Medway DA, Hackney JD (1981) Human exposure to ferric sulfate aerosol: effects on pulmonary function and respiratory symptoms. Am Ind Hyg Assoc J 42:298–304. doi:10.1080/15298668191419767
Kocyigit A, Armutcu F, Gurel A, Ermis B (2004) Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biol Trace Elem Res 97:31–41. doi:10.1385/BTER:97:1:31
Koenig JQ et al (2005) Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect 113:499–503
Komarov AM, Hall JM, Chmielinska JJ, Weglicki WB (2006) Iron uptake and release by macrophages is sensitive to propranolol. Mol Cell Biochem 288:213–217. doi:10.1007/s11010-006-9138-2
Konijn AM, Hershko C (1977) Ferritin synthesis in inflammation. I Pathogenesis of impaired iron release. Br J Haematol 37:7–16
Kougias M et al (2013) The acute effect of cigarette smoking on the respiratory function and FENO production among young smokers. Exp Lung Res 39:359–364. doi:10.3109/01902148.2013.830654
Kozyrskyj AL, Pawlowski AN (2013) Maternal distress and childhood wheeze: mechanisms and context. Am J Respir Crit Care Med 187:1160–1162. doi:10.1164/rccm.201304-0649ED
Krijt J, Vokurka M, Sefc L, Duricova D, Necas E (2006) Effect of lipopolysaccharide and bleeding on the expression of intestinal proteins involved in iron and haem transport. Folia Biol (Praha) 52:1–5
Kruger PC, Leahy MF, Olynyk JK (2012) Assessing iron overload: are we there yet? Clin Cancer Res 18:6395–6397. doi:10.1158/1078-0432.CCR-12-2881
La Corte R, Bajocchi G, Trotta F, Potena A (1992) Bronchial hyperresponsiveness to methacholine in primary Sjogren’s syndrome. Ann Rheum Dis 51:431
La Corte R, Bajocchi G, Potena A, Govoni M, Trotta F (1995) Bronchial hyperreactivity in systemic sclerosis patients: influence of associated Sjogren’s syndrome. Ann Rheum Dis 54:636–639
Landahl G, Adolfsson P, Borjesson M, Mannheimer C, Rodjer S (2005) Iron deficiency and anemia: a common problem in female elite soccer players. Int J Sport Nutr Exerc Metab 15:689–694
Landry JS, Chan T, Lands L, Menzies D (2011) Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J 18:265–270
Landry JS, Tremblay GM, Li PZ, Wong C, Benedetti A, Taivassalo T (2016) Lung function and bronchial hyperresponsiveness in adults born prematurely. A cohort study. Ann Am Thorac Soc 13:17–24. doi:10.1513/AnnalsATS.201508-553OC
Langdeau JB, Turcotte H, Bowie DM, Jobin J, Desgagne P, Boulet LP (2000) Airway hyperresponsiveness in elite athletes. Am J Respir Crit Care Med 161:1479–1484. doi:10.1164/ajrccm.161.5.9909008
Lange NE, Bunyavanich S, Silberg JL, Canino G, Rosner BA, Celedon JC (2011) Parental psychosocial stress and asthma morbidity in Puerto Rican twins. J Allergy Clin Immunol 127(734–740):e731–737. doi:10.1016/j.jaci.2010.11.010
Lapp NL, Hankinson JL, Burgess DB, O’Brien R (1972) Changes in ventilatory function in coal miners after a work shift. Arch Environ Health 24:204–208
Lau S et al (2002) The development of childhood asthma: lessons from the German Multicentre Allergy Study (MAS). Paediatr Respir Rev 3:265–272
Le Souef PN, Sears MR, Sherrill D (1995) The effect of size and age of subject on airway responsiveness in children. Am J Respir Crit Care Med 152:576–579. doi:10.1164/ajrccm.152.2.7633710
Lee JH, Haselkorn T, Chipps BE, Miller DP, Wenzel SE, Tenor Study G (2006) Gender differences in IgE-mediated allergic asthma in the epidemiology and natural history of asthma: Outcomes and Treatment Regimens (TENOR) study. J Asthma 43:179–184. doi:10.1080/02770900600566405
Lee SK et al (2008) Iron chelator differentially activates macrophage inflammatory protein-3alpha/CCL20 in immortalized and malignant human oral keratinocytes. Arch Oral Biol 53:801–809. doi:10.1016/j.archoralbio.2008.01.015
Leon Hsu HH et al (2015) Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med 192:1052–1059. doi:10.1164/rccm.201504-0658OC
Leschziner G, Gringras P (2012) Restless legs syndrome. BMJ 344:e3056. doi:10.1136/bmj.e3056
Letner D, Peloquin J, Durand J, Rutherford A, Yajnik V, Khalili H, Garber J (2015) Elevated total iron-binding capacity is associated with an increased risk of celiac disease. Dig Dis Sci 60:3735–3742. doi:10.1007/s10620-015-3791-9
Levy A, Fraser D, Katz M, Mazor M, Sheiner E (2005) Maternal anemia during pregnancy is an independent risk factor for low birthweight and preterm delivery. Eur J Obstet Gynecol Reprod Biol 122:182–186. doi:10.1016/j.ejogrb.2005.02.015
Leynaert B, Bousquet J, Henry C, Liard R, Neukirch F (1997) Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med 156:1413–1420. doi:10.1164/ajrccm.156.5.9701060
Li J et al (2015) Genome-wide admixture and association study of serum iron, ferritin, transferrin saturation and total iron binding capacity in African Americans. Hum Mol Genet 24:572–581. doi:10.1093/hmg/ddu454
Lieske B, Becker I, Schulz RJ, Polidori MC, Kassubek J, Roehrig G (2015) Intravenous iron administration in restless legs syndrome: an observational study in geriatric patients. Z Gerontol Geriatr. doi:10.1007/s00391-015-0984-y
Liles AM (2012) Intravenous versus oral iron for treatment of iron deficiency in non-hemodialysis-dependent patients with chronic kidney disease. Am J Health Syst Pharm 69:1206–1211. doi:10.2146/ajhp110231
Little JW, Hall WJ, Douglas RG Jr, Mudholkar GS, Speers DM, Patel K (1978) Airway hyperreactivity and peripheral airway dysfunction in influenza A infection. Am Rev Respir Dis 118:295–303
Liu L, Xiao Y, Zou B, Zhao LL (2015) Study of the significance of iron deficiency indexes and erythrocyte parameters in anemic pregnant women and their newborns. Genet Mol Res 14:3501–3508. doi:10.4238/2015.April.15.14
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L (2015) Iron deficiency anaemia. Lancet. doi:10.1016/S0140-6736(15)60865-0
Lounis N, Maslo C, Truffot-Pernot C, Grosset J, Boelaert RJ (2003) Impact of iron loading on the activity of isoniazid or ethambutol in the treatment of murine tuberculosis. Int J Tuberc Lung Dis 7:575–579
Loyo-Berrios NI, Irizarry R, Hennessey JG, Tao XG, Matanoski G (2007) Air pollution sources and childhood asthma attacks in Catano, Puerto Rico. Am J Epidemiol 165:927–935. doi:10.1093/aje/kwk088
Lu H et al (2009) Hypoxia-inducible factor-1alpha blocks differentiation of malignant gliomas. FEBS J 276:7291–7304. doi:10.1111/j.1742-4658.2009.07441.x
Lu FL et al (2012) Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol 176:32–42. doi:10.1093/aje/kwr484
Ludvigsson JF, Hemminki K, Wahlstrom J, Almqvist C (2011) Celiac disease confers a 1.6-fold increased risk of asthma: a nationwide population-based cohort study. J Allergy Clin Immunol 127:1071–1073. doi:10.1016/j.jaci.2010.12.1076
Lundstrom U, Siimes MA, Dallman PR (1977) At what age does iron supplementation become necessary in low-birth-weight infants? J Pediatr 91:878–883
Maazi H, Shirinbak S, Bloksma N, Nawijn MC, van Oosterhout AJ (2011) Iron administration reduces airway hyperreactivity and eosinophilia in a mouse model of allergic asthma. Clin Exp Immunol 166:80–86. doi:10.1111/j.1365-2249.2011.04448.x
Maes M, Van de Vyvere J, Vandoolaeghe E, Bril T, Demedts P, Wauters A, Neels H (1996) Alterations in iron metabolism and the erythron in major depression: further evidence for a chronic inflammatory process. J Affect Disord 40:23–33
Mainous AG 3rd, Diaz VA (2009) Relation of serum ferritin level to cardiovascular fitness among young men. Am J Cardiol 103:115–118. doi:10.1016/j.amjcard.2008.08.046
Mak IT, Chmielinska JJ, Nedelec L, Torres A, Weglicki WB (2006) D-propranolol attenuates lysosomal iron accumulation and oxidative injury in endothelial cells. J Pharmacol Exp Ther 317:522–528. doi:10.1124/jpet.105.097709
Malik IA, Naz N, Sheikh N, Khan S, Moriconi F, Blaschke M, Ramadori G (2011) Comparison of changes in gene expression of transferrin receptor-1 and other iron-regulatory proteins in rat liver and brain during acute-phase response. Cell Tissue Res 344:299–312. doi:10.1007/s00441-011-1152-3
Malo JL, Cartier A, Dolovich J (1993) Occupational asthma due to zinc. Eur Respir J 6:447–450
Manfreda J et al (2001) Prevalence of asthma symptoms among adults aged 20–44 years in Canada. CMAJ 164:995–1001
Manners S, Alam R, Schwartz DA, Gorska MM (2014) A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol 134:63–72. doi:10.1016/j.jaci.2013.10.047
Martin RJ (2006) Infections and asthma. Clin Chest Med 27:87–98, vi. doi:10.1016/j.ccm.2005.10.004
Martinez-Moragon E, Plaza V, Serrano J, Picado C, Galdiz JB, Lopez-Vina A, Sanchis J (2004) Near-fatal asthma related to menstruation. J Allergy Clin Immunol 113:242–244. doi:10.1016/j.jaci.2003.11.002
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483. doi:10.1146/annurev.immunol.021908.132532
Masson C (2011) Rheumatoid anemia. Joint Bone Spine 78:131–137. doi:10.1016/j.jbspin.2010.05.017
Mauad T et al (2008) Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med 178:721–728. doi:10.1164/rccm.200803-436OC
McKay RH, Higuchi DA, Winder WW, Fell RD, Brown EB (1983) Tissue effects of iron deficiency in the rat. Biochim Biophys Acta 757:352–358
McKeever TM, Lewis SA, Smith C, Hubbard R (2002) The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Respir Crit Care Med 166:827–832. doi:10.1164/rccm.200202-158OC
McLaren CE et al (2012) Associations between single nucleotide polymorphisms in iron-related genes and iron status in multiethnic populations. PLoS One 7:e38339. doi:10.1371/journal.pone.0038339
Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS (2007) Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep 7:143–150. doi:10.1007/s11882-007-0012-4
Meng YY, Wilhelm M, Rull RP, English P, Ritz B (2007) Traffic and outdoor air pollution levels near residences and poorly controlled asthma in adults. Ann Allergy Asthma Immunol 98:455–463. doi:10.1016/S1081-1206(10)60760-0
Miller AC, Gladwin MT (2012) Pulmonary complications of sickle cell disease. Am J Respir Crit Care Med 185:1154–1165. doi:10.1164/rccm.201111-2082CI
Miller JM, Sproule BJ (1966) Acute effects of inhalation of cigarette smoke on mechanical properties of the lungs. Am Rev Respir Dis 94:721–726
Milman N, Kaas Ibsen K (1984) Serum ferritin in Danish children and adolescents. Scand J Haematol 33:260–266
Milman N, Agger AO, Nielsen OJ (1991) Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull 38:471–476
Mims MP, Prchal JT (2005) Divalent metal transporter 1. Hematology 10:339–345. doi:10.1080/10245330500093419
Mohanan S, Tapp H, McWilliams A, Dulin M (2014) Obesity and asthma: pathophysiology and implications for diagnosis and management in primary care. Exp Biol Med (Maywood) 239:1531–1540. doi:10.1177/1535370214525302
Mohanty D et al (2008) Iron deficiency anaemia in sickle cell disorders in India. Indian J Med Res 127:366–369
Moon JH, Kim C, Lee HS, Kim SW, Lee JY (2013) Antibacterial and antibiofilm effects of iron chelators against Prevotella intermedia. J Med Microbiol 62:1307–1316. doi:10.1099/jmm.0.053553-0
Moreb J, Hershko C, Hasin Y (1988) Effects of acute iron loading on contractility and spontaneous beating rate of cultured rat myocardial cells. Basic Res Cardiol 83:360–368
Mu M, Ye S, Bai MJ, Liu GL, Tong Y, Wang SF, Sheng J (2014) Birth weight and subsequent risk of asthma: a systematic review and meta-analysis. Heart Lung Circ 23:511–519. doi:10.1016/j.hlc.2013.11.018
Mukhopadhyay K, Yadav RK, Kishore SS, Garewal G, Jain V, Narang A (2011) Iron status at birth and at 4 weeks in term small-for-gestation infants in comparison with appropriate-for-gestation infants. J Matern Fetal Neonatal Med 24:886–890. doi:10.3109/14767058.2010.536866
Murphy VE, Gibson PG (2011) Asthma in pregnancy. Clin Chest Med 32:93–110, ix. doi:10.1016/j.ccm.2010.10.001
Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, Gibson PG (2011) A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 118:1314–1323. doi:10.1111/j.1471-0528.2011.03055.x
Murray JA et al (2013) Association between celiac disease and iron deficiency in Caucasians, but not non-Caucasians. Clin Gastroenterol Hepatol 11:808–814. doi:10.1016/j.cgh.2013.02.009
Naderi N, Etaati Z, Rezvani Joibari M, Sobhani SA, Hosseni Tashnizi S (2013) Immune deviation in recurrent vulvovaginal candidiasis: correlation with iron deficiency anemia. Iran J Immunol 10:118–126. doi:IJIv10i2A7
Nakagawa H et al (2014) Inverse correlation between serum interleukin-6 and iron levels among Japanese adults: a cross-sectional study. BMC Hematol. doi:10.1186/2052-1839-14-6
Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M (2004) Overweight children and adolescents: a risk group for iron deficiency. Pediatrics 114:104–108
Neuman A et al (2012) Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 186:1037–1043. doi:10.1164/rccm.201203-0501OC
Neymotin F, Sen U (2011) Iron and obesity in females in the United States. Obesity (Silver Spring) 19:191–199. doi:10.1038/oby.2010.112
Nwaru BI et al (2014) An exploratory study of the associations between maternal iron status in pregnancy and childhood wheeze and atopy. Br J Nutr 112:2018–2027. doi:10.1017/S0007114514003122
Nyakeriga AM et al (2005) Cytokine mRNA expression and iron status in children living in a malaria endemic area. Scand J Immunol 61:370–375. doi:10.1111/j.1365-3083.2005.01573.x
O’Connor GT, Sparrow D, Segal MR, Weiss ST (1989) Smoking, atopy, and methacholine airway responsiveness among middle-aged and elderly men. The Normative Aging Study. Am Rev Respir Dis 140:1520–1526. doi:10.1164/ajrccm/140.6.1520
O’Connor GT et al (2008) Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol 121(1133–1139):e1131. doi:10.1016/j.jaci.2008.02.020
Ogilvie-Harris DJ, Fornaiser VL (1980) Synovial iron deposition in osteoarthritis and rheumatoid arthritis. J Rheumatol 7:30–36
Olivares M, Walter T, Osorio M, Chadud P, Schlesinger L (1989) Anemia of a mild viral infection: the measles vaccine as a model. Pediatrics 84:851–855
Oliveti JF, Kercsmar CM, Redline S (1996) Pre- and perinatal risk factors for asthma in inner city African-American children. Am J Epidemiol 143:570–577
Orr JS et al (2014) Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 63:421–432. doi:10.2337/db13-0213
Orro T, Sankari S, Pudas T, Oksanen A, Soveri T (2004) Acute phase response in reindeer after challenge with Escherichia coli endotoxin. Comp Immunol Microbiol Infect Dis 27:413–422. doi:10.1016/j.cimid.2004.01.005
Ostojic SM, Ahmetovic Z (2008) Weekly training volume and hematological status in female top-level athletes of different sports. J Sports Med Phys Fitness 48:398–403
Ostransky D, Blais FX (1991) Postviral bronchial hyperreactivity syndrome: recognizing asthma’s great mimic. J Am Osteopath Assoc 91:465–468, 471–465
Overlack A (1996) ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf 15:72–78
Palermo C, Maddali Bongi S, Bianucci G (1986) Relationship between serum ferritin, iron stores and disease activity in rheumatoid arthritis Ric. Clin Lab 16:463–469
Palma-Carlos AG, Palma-Carlos ML, Costa AC (2005) “Minor” hemoglobinopathies: a risk factor for asthma. Eur Ann Allergy Clin Immunol 37:177–182
Pamuk GE et al (2015) Is iron-deficiency anemia associated with migraine? Is there a role for anxiety and depression? Wien Klin Wochenschr. doi:10.1007/s00508-015-0740-8
Papaioannou AI et al (2010) The acute effect of smoking in healthy and asthmatic smokers. Eur J Clin Invest 40:103–109. doi:10.1111/j.1365-2362.2009.02221.x
Pateva IB, Kerling EH, Reddy M, Chen D, Carlson SE, Tancabelic J (2015) Effect of maternal cigarette smoking on newborn iron stores. Clin Res Trials 1:4–7
Pattini A, Schena F, Guidi GC (1990) Serum ferritin and serum iron changes after cross-country and roller ski endurance races. Eur J Appl Physiol Occup Physiol 61:55–60
Pearson VE, Gamaldo CE, Allen RP, Lesage S, Hening WA, Earley CJ (2008) Medication use in patients with restless legs syndrome compared with a control population. Eur J Neurol 15:16–21. doi:10.1111/j.1468-1331.2007.01991.x
Perzanowski MS et al (2006) Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol 117:1082–1089. doi:10.1016/j.jaci.2005.12.1348
Pfeiffer CM, Sternberg MR, Caldwell KL, Pan Y (2013) Race-ethnicity is related to biomarkers of iron and iodine status after adjusting for sociodemographic and lifestyle variables in NHANES 2003–2006. J Nutr 143:977S–985S. doi:10.3945/jn.112.173039
Phillips SF, Fernandez R (1981) Pectin and cellulose binding of iron in vitro. Am J Clin Nutr 34:2322–2323
Phillips AK et al (2014) Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol 34:513–518. doi:10.1038/jp.2014.42
Picchietti DL, Wang VC, Picchietti MA (2012) Intravenous iron given prior to pregnancy for restless legs syndrome is associated with remission of symptoms. J Clin Sleep Med 8:585–586. doi:10.5664/jcsm.2168
Platt SR, Clydesdale FM (1984) Binding of iron by cellulose, lignin, sodium phytate and beta-glucan, alone and in combination, under simulated gastrointestingal pH conditions. J Food Sci 49:531–535
Plessers E, Wyns H, Watteyn A, Pardon B, De Backer P, Croubels S (2015) Characterization of an intravenous lipopolysaccharide inflammation model in calves with respect to the acute-phase response. Vet Immunol Immunopathol 163:46–56. doi:10.1016/j.vetimm.2014.11.005
Postma DS (2007) Gender differences in asthma development and progression. Gender Med 4(Suppl B):S133–S146
Potena A, La Corte R, Fabbri LM, Papi A, Trotta F, Ciaccia A (1990) Increased bronchial responsiveness in primary and secondary Sjogren’s syndrome. Eur Respir J 3:548–553
Prabhu N et al (2010) First trimester maternal tobacco smoking habits and fetal growth. Thorax 65:235–240. doi:10.1136/thx.2009.123232
Puolakka J (1980) Serum ferritin as a measure of iron stores during pregnancy. Acta Obstet Gynecol Scand Suppl 95:1–31
Puolakka J, Janne O, Vihko R (1980) Evaluation by serum ferritin assay of the influence of maternal iron stores on the iron status of newborns and infants. Acta Obstet Gynecol Scand Suppl 95:53–56
Rabinovitch N, Strand M, Gelfand EW (2006) Particulate levels are associated with early asthma worsening in children with persistent disease. Am J Respir Crit Care Med 173:1098–1105. doi:10.1164/rccm.200509-1393OC
Raj D, Kabra SK, Lodha R (2014) Childhood obesity and risk of allergy or asthma. Immunol Allergy Clin North Am 34:753–765. doi:10.1016/j.iac.2014.07.001
Ramakrishnan K, Borade A (2010) Anemia as a risk factor for childhood asthma. Lung India 27:51–53. doi:10.4103/0970-2113.63605
Randolph C (2009) An update on exercise-induced bronchoconstriction with and without asthma. Curr Allergy Asthma Rep 9:433–438
Rao CK et al (2013) Characteristics of perimenstrual asthma and its relation to asthma severity and control: data from the Severe Asthma Research Program. Chest 143:984–992. doi:10.1378/chest.12-0973
Rasmussen K (2001) Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? J Nutr 131:590S–601S; discussion 601S–603S
Reardon TF, Allen DG (2009) Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp Physiol 94:720–730. doi:10.1113/expphysiol.2008.046045
Reinke S et al (2012) Absolute and functional iron deficiency in professional athletes during training and recovery. Int J Cardiol 156:186–191. doi:10.1016/j.ijcard.2010.10.139
Reissman KR, Coleman TJ, Budai BS, Moriarty LR (1955) Acute intestinal iron intoxication. I Iron absorption, serum iron and autopsy findings. Blood 10:35–45
Rennie DC, Lawson JA, Senthilselvan A, Willson PJ, Dosman JA (2012) Domestic endotoxin exposure and asthma in children: epidemiological studies. Front Biosci (Elite Ed) 4:56–73
Rhodes CJ et al (2011) Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol 58:300–309. doi:10.1016/j.jacc.2011.02.057
Ribot B, Aranda N, Viteri F, Hernandez-Martinez C, Canals J, Arija V (2012) Depleted iron stores without anaemia early in pregnancy carries increased risk of lower birthweight even when supplemented daily with moderate iron. Hum Reprod 27:1260–1266. doi:10.1093/humrep/des026
Ribot B, Aranda N, Giralt M, Romeu M, Balaguer A, Arija V (2013) Effect of different doses of iron supplementation during pregnancy on maternal and infant health. Ann Hematol 92:221–229. doi:10.1007/s00277-012-1578-z
Ricciardolo FL, Rado V, Fabbri LM, Sterk PJ, Di Maria GU, Geppetti P (1999) Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am J Respir Crit Care Med 159:557–562. doi:10.1164/ajrccm.159.2.9804022
Ridefelt P, Larsson A, Rehman JU, Axelsson J (2010) Influences of sleep and the circadian rhythm on iron-status indices. Clin Biochem 43:1323–1328. doi:10.1016/j.clinbiochem.2010.08.023
Robotham JL, Lietman PS (1980) Acute iron poisoning. A review. Am J Dis Child 134:875–879
Ronchetti R et al (2002) Association of asthma with extra-respiratory symptoms in schoolchildren: two cross-sectional studies 6 years apart. Pediatr Allergy Immunol 13:113–118
Rosenberg SL, Miller GE, Brehm JM, Celedon JC (2014) Stress and asthma: novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol 134:1009–1015. doi:10.1016/j.jaci.2014.07.005
Ruiter G et al (2011) Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J 37:1386–1391. doi:10.1183/09031936.00100510
Rumpel JA et al (2012) Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol 130:1302–1306. doi:10.1016/j.jaci.2012.09.001
Said SI, Hamidi SA, Gonzalez Bosc L (2010) Asthma and pulmonary arterial hypertension: do they share a key mechanism of pathogenesis? Eur Respir J 35:730–734. doi:10.1183/09031936.00097109
Sauni R, Linna A, Oksa P, Nordman H, Tuppurainen M, Uitti J (2010) Cobalt asthma—a case series from a cobalt plant. Occup Med (Lond) 60:301–306. doi:10.1093/occmed/kqq023
Schatz M, Camargo CA Jr (2003) The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 91:553–558. doi:10.1016/S1081-1206(10)61533-5
Schmidt PJ (2015) Regulation of iron metabolism by hepcidin under conditions of inflammation. J Biol Chem 290:18975–18983. doi:10.1074/jbc.R115.650150
Schneider BC, Constant SL, Patierno SR, Jurjus RA, Ceryak SM (2012) Exposure to particulate hexavalent chromium exacerbates allergic asthma pathology. Toxicol Appl Pharmacol 259:38–44. doi:10.1016/j.taap.2011.12.001
Scholl TO (2005) Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 81:1218S–1222S
Schreinemachers DM, Ghio AJ (2016) Effects of environmental pollutants on cellular iron homeostasis and ultimate links to human disease. Environ Health Insights 10:35–43. doi:10.4137/EHI.S36225
Scott PH, Berger HM, Kenward C, Scott P, Wharton BA (1975) Effect of gestational age and intrauterine nutrition on plasma transferrin and iron in the newborn. Arch Dis Child 50:796–798
Shaheen SO, Newson RB, Henderson AJ, Emmett PM, Sherriff A, Cooke M, Team AS (2004) Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J 24:292–297
Shakur YA, Choudhury N, Hyder SM, Zlotkin SH (2010) Unexpectedly high early prevalence of anaemia in 6-month-old breast-fed infants in rural Bangladesh. Public Health Nutr 13:4–11. doi:10.1017/S1368980009005886
Sheikh N, Dudas J, Ramadori G (2007) Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest 87:713–725. doi:10.1038/labinvest.3700553
Shen TC, Tu CY, Lin CL, Wei CC, Li YF (2014) Increased risk of asthma in patients with systemic lupus erythematosus. Am J Respir Crit Care Med 189:496–499. doi:10.1164/rccm.201310-1792LE
Shields RC, Caric V, Hair M, Jones O, Wark L, McColl MD, Ramsay JE (2011) Pregnancy-specific reference ranges for haematological variables in a Scottish population. J Obstet Gynaecol 31:286–289. doi:10.3109/01443615.2010.545900
Short PM, Williamson PA, Lipworth BJ (2012) Sensitivity of impulse oscillometry and spirometry in beta-blocker induced bronchoconstriction and beta-agonist bronchodilatation in asthma. Ann Allergy Asthma Immunol 109:412–415. doi:10.1016/j.anai.2012.09.010
Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK (2007) The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology 92:73–82. doi:10.1159/000100805
Siddiqui AR, Gold EB, Yang X, Lee K, Brown KH, Bhutta ZA (2008) Prenatal exposure to wood fuel smoke and low birth weight. Environ Health Perspect 116:543–549. doi:10.1289/ehp.10782
Silverberg JI, Hanifin JM (2013) Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol 132:1132–1138. doi:10.1016/j.jaci.2013.08.031
Simonsson BG, Jacobs FM, Nadel JA (1967) Role of autonomic nervous system and the cough reflex in the increased responsiveness of airways in patients with obstructive airway disease. J Clin Invest 46:1812–1818. doi:10.1172/JCI105671
Simpson DL, Goodman M, Spector SL, Petty TL (1978) Long-term follow-up and bronchial reactivity testing in survivors of the adult respiratory distress syndrome. Am Rev Respir Dis 117:449–454
Singhal A, Doherty JF, Raynes JG, McAdam KP, Thomas PW, Serjeant BE, Serjeant GR (1993) Is there an acute-phase response in steady-state sickle cell disease? Lancet 341:651–653
Singla PN, Chand S, Agarwal KN (1979) Cord serum and placental tissue iron status in maternal hypoferremia. Am J Clin Nutr 32:1462–1465
Singla PN, Tyagi M, Kumar A, Dash D, Shankar R (1997) Fetal growth in maternal anaemia. J Trop Pediatr 43:89–92
Singleton RJ, Holman RC, Cobb N, Curns AT, Paisano EL (2006) Asthma hospitalizations among American Indian and Alaska Native people and for the general US population. Chest 130:1554–1562. doi:10.1378/chest.130.5.1554
Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM (1992) The influence of age and sex on asthma admissions. JAMA 268:3437–3440
Skrzypczak W, Dratwa-Chalupnik A, Ozgo M, Michalek K, Lepczynski A, Hejza K, Siwa J (2010) Effect of converting enzyme inhibitor on copper and iron concentrations of blood plasma in calves during the neonatal period. Folia Biol (Krakow) 58:119–124
Smith DJ, Roberts D (1994) Effects of high volume and/or intense exercise on selected blood chemistry parameters. Clin Biochem 27:435–440
Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, Robbins PA (2008) The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol 586:5999–6005. doi:10.1113/jphysiol.2008.160960
Smith TG et al (2009) Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA 302:1444–1450. doi:10.1001/jama.2009.1404
Sobke A et al (2012) The urinary antibiotic 5-nitro-8-hydroxyquinoline (Nitroxoline) reduces the formation and induces the dispersal of Pseudomonas aeruginosa biofilms by chelation of iron and zinc. Antimicrob Agents Chemother 56:6021–6025. doi:10.1128/AAC.01484-12
Sobol BJ, Van Voorhies L, Emirgil C (1977) Detection of acute effects of cigarette smoking on airway dynamics: a critical and comparative study of pulmonary function tests. Thorax 32:312–316
Solis JV et al (2006) Iron deficiency in the acute-phase reaction after open aortic surgery. Vasc Endovasc Surg 40:392–398. doi:10.1177/1538574406293749
Sorkness R, Clough JJ, Castleman WL, Lemanske RF Jr (1994) Virus-induced airway obstruction and parasympathetic hyperresponsiveness in adult rats. Am J Respir Crit Care Med 150:28–34. doi:10.1164/ajrccm.150.1.8025764
Spencer H (1985) Pathology of the Lung, 4th edn. Pergamon Press, New York
Spengler CM, Shea SA (2000) Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med 162:1038–1046. doi:10.1164/ajrccm.162.3.9911107
Stein RT, Holberg CJ, Sherrill D, Wright AL, Morgan WJ, Taussig L, Martinez FD (1999) Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol 149:1030–1037
Stieb DM, Chen L, Eshoul M, Judek S (2012) Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 117:100–111. doi:10.1016/j.envres.2012.05.007
Stokka GL, Edwards AJ, Spire MF, Brandt RT Jr, Smith JE (1994) Inflammatory response to clostridial vaccines in feedlot cattle. J Am Vet Med Assoc 204:415–419
Strunk RC, Brown MS, Boyd JH, Bates P, Field JJ, DeBaun MR (2008) Methacholine challenge in children with sickle cell disease: a case series. Pediatr Pulmonol 43:924–929. doi:10.1002/ppul.20884
Sue-Chu M (2012) Winter sports athletes: long-term effects of cold air exposure. Br J Sports Med 46:397–401. doi:10.1136/bjsports-2011-090822
Sukumaran A et al (2014) Expression of iron-related proteins in the duodenum is up-regulated in patients with chronic inflammatory disorders. Br J Nutr 111:1059–1068. doi:10.1017/S0007114513003334
Sundblad BM, Larsson BM, Palmberg L, Larsson K (2002) Exhaled nitric oxide and bronchial responsiveness in healthy subjects exposed to organic dust. Eur Respir J 20:426–431
Sunyer J, Anto JM, Kogevinas M, Soriano JB, Tobias A, Munoz A (1997) Smoking and bronchial responsiveness in nonatopic and atopic young adults. Spanish Group of the European Study of Asthma. Thorax 52:235–238
Sutherland ER (2005) Nocturnal asthma. J Allergy Clin Immunol 116:1179–1186; quiz 1187. doi:10.1016/j.jaci.2005.09.028
Takala TI et al (2010) Blood cell and iron status analytes of preterm and full-term infants from 20 weeks onwards during the first year of life. Clin Chem Lab Med 48:1295–1301. doi:10.1515/CCLM.2010.242
Tang YJ, Wang K, Yuan T, Qiu T, Xiao J, Yi Q, Feng YL (2010) Salmeterol/fluticasone treatment reduces circulating C-reactive protein level in patients with stable chronic obstructive pulmonary disease. Chin Med J (Engl) 123:1652–1657
Tapryal N, Vivek GV, Mukhopadhyay CK (2015) Catecholamine stress hormones regulate cellular iron homeostasis by a posttranscriptional mechanism mediated by iron regulatory protein: implication in energy homeostasis. J Biol Chem 290:7634–7646. doi:10.1074/jbc.M114.592519
Tashkin DP, Altose MD, Bleecker ER, Connett JE, Kanner RE, Lee WW, Wise R (1992) The lung health study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am Rev Respir Dis 145:301–310. doi:10.1164/ajrccm/145.2_Pt_1.301
Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ Jr, Zeldin DC (2005) Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med 172:1371–1377. doi:10.1164/rccm.200505-758OC
Thorne PS et al (2015) Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med 192:1287–1297. doi:10.1164/rccm.201502-0251OC
Tirumalasetty J, Grammer LC (2006) Asthma, surgery, and general anesthesia: a review. J Asthma 43:251–254. doi:10.1080/02770900600643162
Tough SC, Hessel PA, Ruff M, Green FH, Mitchell I, Butt JC (1998) Features that distinguish those who die from asthma from community controls with asthma. J Asthma 35:657–665
Trenga CA et al (2006) Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest 129:1614–1622. doi:10.1378/chest.129.6.1614
Triche EW, Lundsberg LS, Wickner PG, Belanger K, Leaderer BP, Bracken MB (2011) Association of maternal anemia with increased wheeze and asthma in children. Ann Allergy Asthma Immunol 106(131–139):e131. doi:10.1016/j.anai.2010.11.007
Trost LB, Bergfeld WF, Calogeras E (2006) The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol 54:824–844. doi:10.1016/j.jaad.2005.11.1104
Uijterschout L et al (2015) Iron deficiency in the first 6 months of age in infants born between 32 and 37 weeks of gestational age. Eur J Clin Nutr 69:598–602. doi:10.1038/ejcn.2014.217
Unger EL, Earley CJ, Beard JL (2009) Diurnal cycle influences peripheral and brain iron levels in mice. J Appl Physiol (1985) 106:187–193. doi:10.1152/japplphysiol.91076.2008
Unger EL, Jones BC, Bianco LE, Allen RP, Earley CJ (2014) Diurnal variations in brain iron concentrations in BXD RI mice. Neuroscience 263:54–59. doi:10.1016/j.neuroscience.2013.12.056
van Delden C, Page MG, Kohler T (2013) Involvement of Fe uptake systems and AmpC beta-lactamase in susceptibility to the siderophore monosulfactam BAL30072 in Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:2095–2102. doi:10.1128/AAC.02474-12
van Steenbergen HW, van Nies JA, van der Helm-van Mil AH (2013) Anaemia to predict radiographic progression in rheumatoid arthritis. Ann Rheum Dis 72:e16. doi:10.1136/annrheumdis-2013-203718
Vanarsa K, Ye Y, Han J, Xie C, Mohan C, Wu T (2012) Inflammation associated anemia and ferritin as disease markers in SLE. Arthritis Res Ther 14:R182. doi:10.1186/ar4012
Varesio L, Battaglia F, Raggi F, Ledda B, Bosco MC (2010) Macrophage-inflammatory protein-3alpha/CCL-20 is transcriptionally induced by the iron chelator desferrioxamine in human mononuclear phagocytes through nuclear factor (NF)-kappaB. Mol Immunol 47:685–693. doi:10.1016/j.molimm.2009.10.031
Velazquez AM, Christiani DC, McConnell R, Eisen EA, Wilcox M (1991) Respiratory disease in a textile factory in Nicaragua. Am J Ind Med 20:195–208
Vernooy JH et al (2005) Rapid pulmonary expression of acute-phase reactants after local lipopolysaccharide exposure in mice is followed by an interleukin-6 mediated systemic acute-phase response. Exp Lung Res 31:855–871. doi:10.1080/01902140600611645
Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM (2010) Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol 126(498–504):e491–496. doi:10.1016/j.jaci.2010.06.018
Vollsaeter M, Clemm HH, Satrell E, Eide GE, Roksund OD, Markestad T, Halvorsen T (2015) Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Ann Am Thorac Soc 12:313–322. doi:10.1513/AnnalsATS.201406-285OC
Vrieze A, Postma DS, Kerstjens HA (2003) Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 112:271–282
Vukovic-Cvetkovic V, Plavec D, Lovrencic-Huzjan A, Galinovic I, Seric V, Demarin V (2010) Is iron deficiency anemia related to menstrual migraine? Post hoc analysis of an observational study evaluating clinical characteristics of patients with menstrual migraine. Acta Clin Croat 49:389–394
Vural H, Uzun K, Uz E, Kocyigit A, Cigli A, Akyol O (2000) Concentrations of copper, zinc and various elements in serum of patients with bronchial asthma. J Trace Elem Med Biol 14:88–91. doi:10.1016/S0946-672X(00)80036-X
Wallander ML, Leibold EA, Eisenstein RS (2006) Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim Biophys Acta 1763:668–689. doi:10.1016/j.bbamcr.2006.05.004
Wang SJ, Chen PK, Fuh JL (2010) Comorbidities of migraine. Front Neurol 1:16. doi:10.3389/fneur.2010.00016
Weigert R, Dosch NC, Bacsik-Campbell ME, Guilbert TW, Coe CL, Kling PJ (2015) Maternal pregnancy weight gain and cord blood iron status are associated with eosinophilia in infancy. J Perinatol 35:621–626. doi:10.1038/jp.2015.21
Weiler JM et al (2007) American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J Allergy Clin Immunol 119:1349–1358. doi:10.1016/j.jaci.2007.02.041
Weinberg ED (1996) Iron withholding: a defense against viral infections. Biometals 9:393–399
Whitten CF, Brough AJ (1971) The pathophysiology of acute iron poisoning. Clin Toxicol 4:585–595. doi:10.3109/15563657108990981
Whitten CF, Chen YC, Gibson GW (1968) Studies in acute iron poisoning. 3 The hemodynamic alterations in acute experimental iron poisoning. Pediatr Res 2:479–485. doi:10.1203/00006450-196811000-00005
Wilkinson JG, Martin DT, Adams AA, Liebman M (2002) Iron status in cyclists during high-intensity interval training and recovery. Int J Sports Med 23:544–548. doi:10.1055/s-2002-35528
Windham GC, Hopkins B, Fenster L, Swan SH (2000) Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology 11:427–433
Wise J (2013) Daily iron during pregnancy improves birth weight. BMJ 346:f3997. doi:10.1136/bmj.f3997
Wiskin AE, Fleming BJ, Wootton SA, Beattie RM (2012) Anaemia and iron deficiency in children with inflammatory bowel disease J Crohns Colitis 6:687–691. doi:10.1016/j.crohns.2011.12.001
Wittczak T, Dudek W, Krakowiak A, Walusiak J, Palczynski C (2008) Occupational asthma due to manganese exposure: a case report. Int J Occup Med Environ Health 21:81–83. doi:10.2478/v10001-007-0041-1
Wright RJ (2011) Epidemiology of stress and asthma: from constricting communities and fragile families to epigenetics. Immunol Allergy Clin North Am 31:19–39. doi:10.1016/j.iac.2010.09.011
Wu CH, Eren E, Ardern-Jones MR, Venter C (2015) Association between micronutrient levels and chronic spontaneous urticaria. Biomed Res Int 2015:926167. doi:10.1155/2015/926167
Xepapadaki P, Papadopoulos NG, Bossios A, Manoussakis E, Manousakas T, Saxoni-Papageorgiou P (2005) Duration of postviral airway hyperresponsiveness in children with asthma: effect of atopy. J Allergy Clin Immunol 116:299–304. doi:10.1016/j.jaci.2005.04.007
Xepapadaki P, Manios Y, Liarigkovinos T, Grammatikaki E, Douladiris N, Kortsalioudaki C, Papadopoulos NG (2009) Association of passive exposure of pregnant women to environmental tobacco smoke with asthma symptoms in children. Pediatr Allergy Immunol 20:423–429. doi:10.1111/j.1399-3038.2008.00820.x
Yu Y, Richardson DR (2011) Cellular iron depletion stimulates the JNK and p38 MAPK signaling transduction pathways, dissociation of ASK1-thioredoxin, and activation of ASK1. J Biol Chem 286:15413–15427. doi:10.1074/jbc.M111.225946
Yu Y, Kovacevic Z, Richardson DR (2007) Tuning cell cycle regulation with an iron key. Cell Cycle 6:1982–1994
Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR (2011) Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226:2925–2933. doi:10.1002/jcp.22640
Yun HD et al (2012) Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clin Proc 87:953–960. doi:10.1016/j.mayocp.2012.05.020
Zamora TG, Guiang SF, Georgieff MK, Widness JA (2016) Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res. doi:10.1038/pr.2016.20
Zhang J, Stanton DM, Nguyen XV, Liu M, Zhang Z, Gash D, Bing G (2005) Intrapallidal lipopolysaccharide injection increases iron and ferritin levels in glia of the rat substantia nigra and induces locomotor deficits. Neuroscience 135:829–838. doi:10.1016/j.neuroscience.2005.06.049
Zhang HY, Wang ND, Song N, Xu HM, Shi LM, Jiang H, Xie JX (2013) 6-Hydroxydopamine promotes iron traffic in primary cultured astrocytes. Biometals 26:705–714. doi:10.1007/s10534-013-9647-x
Acknowledgments
I thank Dr. Steve Tilley, Joleen Soukup, Lisa Dailey, and Jackie Stonehuerner for review of the manuscript and corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghio, A.J. Asthma as a disruption in iron homeostasis. Biometals 29, 751–779 (2016). https://doi.org/10.1007/s10534-016-9948-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9948-y