Abstract
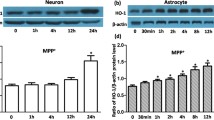
It is well known that disrupted brain iron homeostasis was involved in Parkinson’s disease. We previously reported 6-hydroxydopamine (6-OHDA) could enhance iron influx and attenuate iron efflux process, thus promote iron accumulation in neurons. Astrocytes, the major glial cell type in the central nervous system, are largely responsible for iron distribution in the brain. However, how iron metabolism changes in astrocytes with 6-OHDA treatment are not fully elucidated. In the present study, we first observed that both iron influx and efflux were enhanced with 10 μM 6-OHDA treatment for 24 h in primary cultured astrocytes. In accordance with these iron traffic modulations, both mRNA and protein levels of iron importer divalent metal transporter 1 with iron responsive element (DMT1+IRE) and exporter ferroportin 1 (FPN1) were up-regulated in these cells. L-ferritin mRNA levels were increased. Iron regulatory protein 1 (IRP1) showed a dynamic regulation with 6-OHDA treatment, as indicated by a moderate up-regulation at 12 h, however, down-regulation at 24 h. We further demonstrated that 6-OHDA treatment could induce activation of nuclear factor-kappaB (NF-κB) p65. IκBα activation inhibitor BAY11-7082 fully blocked 6-OHDA induced NF-κB p65 phosphorylation and DMT1 + IRE up-regulation. These results suggest that 6-OHDA might promote iron transport rate in astrocytes by regulating iron transporters, IRP1 expression and NF-κB p65 activation, indicating a different response between neurons and astrocytes.




Similar content being viewed by others
References
Barros LF, Deitmer JW (2010) Glucose and lactate supply to the synapse. Brain Res Rev 63:149–159
Berg D, Youdim MB (2006) Role of iron in neurodegenerative disorders. Top Magn Reson Imaging 17:5–17
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Cairo G, Recalcati S (2007) Iron-regulatory proteins: molecular biology and pathophysiological implications. Expert Rev Mol Med 9:1–13
Christova T, Templeton DM (2007) Effect of hypoxia on the binding and subcellular distribution of iron regulatory proteins. Mol Cell Biochem 301:21–32
Deitmer JW, Rose CR (2010) Ion changes and signalling in perisynaptic glia. Brain Res Rev 63:113–129
Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32:1884–1890
Eisenstein RS (2000) Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr 20:627–662
Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont C, Cabantchik ZI (1999) H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood 94:3593–3603
Fillebeen C, Pantopoulos K (2002) Redox control of iron regulatory proteins. Redox Rep 7:15–22
Galazka-Friedman J, Bauminger ER, Szlachta K, Friedman A (2012) The role of iron in neurodegeneration–Mossbauer spectroscopy, electron microscopy, enzyme-linked immunosorbent assay and neuroimaging studies. J Phys Condens Matter 24(24):244106
Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA (2001) Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 509:309–316
Han J, Day JR, Connor JR, Beard JL (2002) H and L ferritin subunit mRNA expression differs in brains of control and iron-deficient rats. J Nutr 132:2769–2774
Heneka MT, Rodriguez JJ, Verkhratsky A (2010) Neuroglia in neurodegeneration. Brain Res Rev 63:189–211
Hornykiewicz O (1998) Biochemical aspects of Parkinson’s disease. Neurology 51:S2–S9
Janssen-Heininger YM, Macara I, Mossman BT (1999) Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol 20:942–952
Jiang H, Song N, Wang J, Ren LY, Xie JX (2007) Peripheral iron dextran induced degeneration of dopaminergic neurons in rat substantia nigra. Neurochem Int 51:32–36
Jiang H, Song N, Xu H, Zhang S, Wang J, Xie J (2010) Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res 20:345–356
Lee PL, Gelbart T, West C, Halloran C, Beutler E (1998) The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol Dis 24:199–215
Maragakis NJ, Rothstein JD (2006) Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2:679–689
McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902
Molina-Holgado F, Hider RC, Gaeta A, Williams R, Francis P (2007) Metals ions and neurodegeneration. Biometals 20:639–654
Mutze S, Hebling U, Stremmel W, Wang J, Arnhold J, Pantopoulos K, Mueller S (2003) Myeloperoxidase-derived hypochlorous acid antagonizes the oxidative stress-mediated activation of iron regulatory protein 1. J Biol Chem 278:40542–40549
Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D (2005) Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock 23:186–193
Paradkar PN, Roth JA (2006a) Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-kappaB. J Neurochem 96:1768–1777
Paradkar PN, Roth JA (2006b) Post-translational and transcriptional regulation of DMT1 during P19 embryonic carcinoma cell differentiation by retinoic acid. Biochem J 394:173–183
Patel BN, David S (1997) A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem 272:20185–20190
Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, MacNee W (2001) Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-kappaB. Biochem Pharmacol 62:787–794
Schipper HM (1999) Glial HO-1 expression, iron deposition and oxidative stress in neurodegenerative diseases. Neurotox Res 1:57–70
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Song N, Wang J, Jiang H, Xie J (2010) Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinson’s disease. Free Radic Biol Med 48:332–341
Teismann P, Schulz JB (2004) Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res 318:149–161
Tiffany-Castiglion E, Qian Y (2001) Astroglia as metal depots: molecular mechanisms for metal accumulation, storage and release. Neurotoxicology 22:577–592
Tower DB, Young OM (1973) The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem 20:269–278
Tsuji Y, Ayaki H, Whitman SP, Morrow CS, Torti SV, Torti FM (2000) Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol Cell Biol 20:5818–5827
Wang J, Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434:365–381
Wang J, Jiang H, Xie JX (2007) Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats. Eur J Neurosci 25:2766–2772
Xu H, Jiang H, Wang J, Xie J (2010) Rg1 protects the MPP + -treated MES23.5 cells via attenuating DMT1 up-regulation and cellular iron uptake. Neuropharmacology 58:488–494
Youdim MB, Stephenson G, Ben Shachar D (2004) Ironing iron out in Parkinson’s disease and other neurodegenerative diseases with iron chelators: a lesson from 6-hydroxydopamine and iron chelators, desferal and VK-28. Ann N Y Acad Sci 1012:306–325
Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M, Zadlo A, Szewczyk G, Zareba M, Sarna T (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem 106:1866–1875
Zhang S, Wang J, Song N, Xie J, Jiang H (2009) Up-regulation of divalent metal transporter 1 is involved in 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. Neurobiol Aging 30:1466–1476
Acknowledgments
This work was supported by grants from the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2011CB504102, 2012CB526703), the National Nature Science Foundation of China (30930036) and the Department of Science and Technology of Shandong Province (ZR2012HZ005, ZR2010HM003, BS2010SW009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hao-Yun Zhang and Nai-Dong Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, HY., Wang, ND., Song, N. et al. 6-Hydroxydopamine promotes iron traffic in primary cultured astrocytes. Biometals 26, 705–714 (2013). https://doi.org/10.1007/s10534-013-9647-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9647-x