Abstract
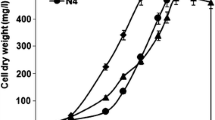
In Pseudomonas aeruginosa the response to oxidative stress is orchestrated by the LysR regulator OxyR by activation of the transcription of two catalase genes (katA and katB), of the alkyl-hydroxyperoxidases ahpCF and ahpB. Next to the expected high sensitivity to oxidative stress generated by reactive oxygen species (ROS: H2O2, O2 −), the oxyR mutant shows a defective growth under conditions of iron limitation (Vinckx et al. 2008). Although production and uptake of the siderophore pyoverdine is not affected by the absence of oxyR, the mutant is unable to satisfy its need for iron when grown under iron limiting conditions. In order to get a better insight into the effects caused by iron limitation on the physiological response of the oxyR mutant we decided to compare the proteomes of the wild type and the mutant grown in the iron-poor casamino acids medium (CAA), in CAA plus H2O2, and in CAA plus the strong iron chelator ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) (EDDHA). Especially in the presence of hydrogen peroxide the oxyR cells increase the production of stress proteins (Dps and IbpA). The superoxide dismutase SodM is produced in higher amounts in the oxyR mutant grown in CAA plus H2O2. The PchB protein, a isochorismate-pyruvate lyase involved in the siderophore pyochelin biosynthesis is not detectable in the extracts from the oxyR mutant grown in the presence of hydrogen peroxide. When cells were grown in the presence of EDDHA, we observed a reduction of the ferric uptake regulator (Fur), and an increase in the two subunits of the succinyl-CoA synthetase and the fumarase FumC1.




Similar content being viewed by others
References
Andrews SC (2010) The ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim Biophys Acta 1800:691–705
Atichartpongkul S, Loprasert S, Vattanaviboon P, Whangsuk W, Helmann JD, Mongkolsuk S (2001) Bacterial Ohr and OsmC paralogues define two protein families with distinct functions and patterns of expression. Microbiology 147:1775–1782
Atichartpongkul S, Fuangthong M, Vattanaviboon P, Mongkolsuk S (2010) Analyses of the regulatory mechanism and physiological roles of Pseudomonas aeruginosa OhrR, a transcription regulator and a sensor of organic hydroperoxides. J Bacteriol 192:2093–2101
Baysse C et al (2000) Vanadium interferes with siderophore-mediated iron uptake in Pseudomonas aeruginosa. Microbiology 146:2425–2434
Bellapadrona G, Ardini M, Ceci P, Stefanini S, Chiancone E (2010) Dps proteins prevent Fenton-mediated oxidative damage by trapping hydroxyl radicals within the protein shell. Free Radic Biol Med 48:292–297
Britigan BE, Roeder TL, Rasmussen GT, Shasby DM, McCormick ML, Cox CD (1992) Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for Pseudomonas-associated tissue injury. J Clin Invest 90:2187–2196
Chattoraj P, Banerjee A, Biswas S, Biswas I (2010) ClpP of Streptococcus mutans differentially regulates expression of genomic islands, mutacin production, and antibiotic tolerance. J Bacteriol 192:1312–1323
Chiancone E, Ceci P (2010) The multifaceted capacity of Dps proteins to combat bacterial stress conditions: detoxification of iron and hydrogen peroxide and DNA binding. Biochim Biophys Acta 1800:798–805
Cornelis P et al (1992) Stability, frequency and multiplicity of transposon insertions in the pyoverdine region in the chromosomes of different fluorescent pseudomonads. J Gen Microbiol 138:1337–1343
Cornelis P, Matthijs S, Van Oeffelen L (2009) Iron uptake regulation in Pseudomonas aeruginosa. Biometals 22:15–22
de Bruijn I, Raaijmakers JM (2009) Regulation of cyclic lipopeptide biosynthesis in Pseudomonas fluorescens by the ClpP protease. J Bacteriol 191:1910–1923
Diggle SP, Stacey RE, Dodd C, Camara M, Williams P, Winzer K (2006) The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ Microbiol 8:1095–1104
Dumont D et al (2007) Characterization of mature rat oligodendrocytes: a proteomic approach. J Neurochem 102:562–576
Escolar L, Perez-Martin J, de Lorenzo V (1999) Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol 181:6223–6229
Fernandes AP, Holmgren A (2004) Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6:63–74
Gaille C, Kast P, Haas D (2002) Salicylate biosynthesis in Pseudomonas aeruginosa. Purification and characterization of PchB, a novel bifunctional enzyme displaying isochorismate pyruvate-lyase and chorismate mutase activities. J Biol Chem 277:21768–21775
Hassett DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177:6330–6337
Hassett DJ, Howell ML, Ochsner UA, Vasil ML, Johnson Z, Dean GE (1997) An operon containing fumC and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J Bacteriol 179:1452–1459
Hassett DJ, Alsabbagh E, Parvatiyar K, Howell ML, Wilmott RW, Ochsner UA (2000) A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J Bacteriol 182:4557–4563
Heo YJ et al (2010) The major catalase gene (katA) of Pseudomonas aeruginosa PA14 is under both positive and negative control of the global transactivator OxyR in response to hydrogen peroxide. J Bacteriol 192:381–390
Hsu JL, Chen HC, Peng HL, Chang HY (2008) Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J Biol Chem 283:9933–9944
Imlay JA (2002) How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis. Adv Microb Physiol 46:111–153
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Kapatral V, Bina X, Chakrabarty AM (2000) Succinyl coenzyme A synthetase of Pseudomonas aeruginosa with a broad specificity for nucleoside triphosphate (NTP) synthesis modulates specificity for NTP synthesis by the 12-kilodalton form of nucleoside diphosphate kinase. J Bacteriol 182:1333–1339
Kirkeby S, Hansen AK, d’ Apice A, Moe D (2006) The galactophilic lectin (PA-IL, gene LecA) from Pseudomonas aeruginosa. Its binding requirements and the localization of lectin receptors in various mouse tissues. Microb Pathog 40:191–197
Kitagawa M, Matsumura Y, Tsuchido T (2000) Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli. FEMS Microbiol Lett 184:165–171
Kitagawa M, Miyakawa M, Matsumura Y, Tsuchido T (2002) Escherichia coli small heat shock proteins, IbpA and IbpB, protect enzymes from inactivation by heat and oxidants. Eur J Biochem 269:2907–2917
Laskowska E, Wawrzynow A, Taylor A (1996) IbpA and IbpB, the new heat-shock proteins, bind to endogenous Escherichia coli proteins aggregated intracellularly by heat shock. Biochimie 78:117–122
Lau GW, Britigan BE, Hassett DJ (2005) Pseudomonas aeruginosa OxyR is required for full virulence in rodent and insect models of infection and for resistance to human neutrophils. Infect Immun 73:2550–2553
Lemire J, Mailloux R, Auger C, Whalen D, Appanna VD (2010) Pseudomonas fluorescens orchestrates a fine metabolic-balancing act to counter aluminium toxicity. Environ Microbiol 12:1384–1390
Lesniak J, Barton WA, Nikolov DB (2003) Structural and functional features of the Escherichia coli hydroperoxide resistance protein OsmC. Protein Sci 12:2838–2843
Li XH et al (2010) The ClpP protease homologue is required for the transmission traits and cell division of the pathogen Legionella pneumophila. BMC Microbiol 10:54
Loughlin MF, Arandhara V, Okolie C, Aldsworth TG, Jenks PJ (2009) Helicobacter pylori mutants defective in the clpP ATP-dependant protease and the chaperone clpA display reduced macrophage and murine survival. Microb Pathog 46:53–57
Marquart ME, Caballero AR, Chomnawang M, Thibodeaux BA, Twining SS, O’Callaghan RJ (2005) Identification of a novel secreted protease from Pseudomonas aeruginosa that causes corneal erosions. Invest Ophthalmol Vis Sci 46:3761–3768
Matuszewska E, Kwiatkowska J, Kuczynska-Wisnik D, Laskowska E (2008) Escherichia coli heat-shock proteins IbpA/B are involved in resistance to oxidative stress induced by copper. Microbiology 154:1739–1747
Melstrom KA Jr et al (2007) Cytotoxicity of Pseudomonas secreted exotoxins requires OxyR expression. J Surg Res 143:50–57
Michel A et al (2006) Global regulatory impact of ClpP protease of Staphylococcus aureus on regulons involved in virulence, oxidative stress response, autolysis, and DNA repair. J Bacteriol 188:5783–5796
Mougous JD et al (2006) A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530
Nystrom T (2005) Role of oxidative carbonylation in protein quality control and senescence. EMBO J 24:1311–1317
Ochsner UA, Vasil ML, Alsabbagh E, Parvatiyar K, Hassett DJ (2000) Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J Bacteriol 182:4533–4544
Panmanee W, Gomez F, Witte D, Pancholi V, Britigan BE, Hassett DJ (2008) The peptidoglycan-associated lipoprotein OprL helps protect a Pseudomonas aeruginosa mutant devoid of the transactivator OxyR from hydrogen peroxide-mediated killing during planktonic and biofilm culture. J Bacteriol 190:3658–3669
Park CY et al (2010) Virulence attenuation of Streptococcus pneumoniae clpP mutant by sensitivity to oxidative stress in macrophages via an NO-mediated pathway. J Microbiol 48:229–235
Parvatiyar K et al (2005) Global analysis of cellular factors and responses involved in Pseudomonas aeruginosa resistance to arsenite. J Bacteriol 187:4853–4864
Ratajczak E, Zietkiewicz S, Liberek K (2009) Distinct activities of Escherichia coli small heat shock proteins IbpA and IbpB promote efficient protein disaggregation. J Mol Biol 386:178–189
Rivera SL, Vargas E, Ramirez-Diaz MI, Campos-Garcia J, Cervantes C (2008) Genes related to chromate resistance by Pseudomonas aeruginosa PAO1. Antonie Van Leeuwenhoek 94:299–305
Serino L, Reimmann C, Baur H, Beyeler M, Visca P, Haas D (1995) Structural genes for salicylate biosynthesis from chorismate in Pseudomonas aeruginosa. Mol Gen Genet 249:217–228
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860
Shouldice SR, Heras B, Jarrott R, Sharma P, Scanlon MJ, Martin JL (2010) Characterization of the DsbA oxidative folding catalyst from Pseudomonas aeruginosa reveals a highly oxidizing protein that binds small molecules. Antioxid Redox Signal 12:921–931
Storz G, Imlay JA (1999) Oxidative stress. Curr Opin Microbiol 2:188–194
Tang A et al (2009) Properties of PASP: a Pseudomonas protease capable of mediating corneal erosions. Invest Ophthalmol Vis Sci 50:3794–3801
Tao K (1997) OxyR-dependent induction of Escherichia coli grx gene expression by peroxide stress. J Bacteriol 179:5967–5970
Teitzel GM, Geddie A, De Long SK, Kirisits MJ, Whiteley M, Parsek MR (2006) Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J Bacteriol 188:7242–7256
Ueda M, Kinoshita H, Maeda SI, Zou W, Tanaka A (2003) Structure-function study of the amino-terminal stretch of the catalase subunit molecule in oligomerization, heme binding, and activity expression. Appl Microbiol Biotechnol 61:488–494
van Oeffelen L, Cornelis P, Van Delm W, De Ridder F, De Moor B, Moreau Y (2008) Detecting cis-regulatory binding sites for cooperatively binding proteins. Nucleic Acids Res 36:e46
Varghese S, Wu A, Park S, Imlay KR, Imlay JA (2007) Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol 64:822–830
Vinckx T, Matthijs S, Cornelis P (2008) Loss of the oxidative stress regulator OxyR in Pseudomonas aeruginosa PAO1 impairs growth under iron-limited conditions. FEMS Microbiol Lett 288:258–265
Vinckx T, Wei Q, Matthijs S, Cornelis P (2010) The Pseudomonas aeruginosa oxidative stress regulator OxyR influences production of pyocyanin and rhamnolipids: protective role of pyocyanin. Microbiology 156:678–686
Yamano Y, Nishikawa T, Komatsu Y (1998) Involvement of the RpoN protein in the transcription of the oprE gene in Pseudomonas aeruginosa. FEMS Microbiol Lett 162:31–37
Yim HH, Villarejo M (1992) osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in Escherichia coli. J Bacteriol 174:3637–3644
Yim HH, Brems RL, Villarejo M (1994) Molecular characterization of the promoter of osmY, an rpoS-dependent gene. J Bacteriol 176:100–107
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinckx, T., Wei, Q., Matthijs, S. et al. A proteome analysis of the response of a Pseudomonas aeruginosa oxyR mutant to iron limitation. Biometals 24, 523–532 (2011). https://doi.org/10.1007/s10534-010-9403-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-010-9403-4