Abstract
Pulses of plant-available nutrients to the soil solution are expected to occur during the dynamic winter–spring transition in arctic tundra. Our aims were to quantify the magnitude of these potential nutrient pulses, to understand the sensitivity of these pulses to winter conditions, and to characterize and integrate the environmental and biogeochemical dynamics of this period. To test the hypotheses that snow depth, temperature and soil water—and not snow nutrient content—are important controls on winter and spring biogeochemistry, we sampled soil from under ambient and deepened snow every 3 days from late winter to spring, in addition to the snowpack at the start of thaw. Soil and microbial biogeochemical dynamics were divided into distinct phases that correlated with steps in soil temperature and soil water. Soil solution and microbial pools of C, N and P fluctuated with strong peaks and declines throughout the thaw, especially under deepened snow. Snowpack nutrient accumulation was negligible relative to these biogeochemical peaks. All nutrient and microbial peaks declined simultaneously at the end of snowmelt and so this decline was delayed by 15 days under deepened snow. The timing of these nutrient pulses is critical for plant species nutrient availability and landscape nutrient budgets. This detailed and statistically-based characterisation of the winter–spring transition in terms of environmental and biogeochemical variables should provide a useful foundation for future biogeochemical process-based studies of thaw, and indicate that spring thaw and possibly growing season biogeochemical dynamics are sensitive to present and future variability in winter snow depth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spring thaw is a period of dynamic transition in arctic ecosystems. The melting snow pack, poor drainage, and thawing active layer result in a temporary period of complete water saturation in many surface soils. The sudden change from cold and dry to warm, moist and relatively anaerobic conditions in the soil environment is stressful to soil microbial organisms and to the physico-chemical structure of soil. These stresses may promote nutrient releases from microbes and from litter and soil organic matter into the soil solution (Chapin et al. 1978; Brooks et al. 1998; Schimel et al. 2007). This spring pulse of nutrient release could be an important control on plant community composition by favouring those plant species that can preferentially acquire nutrients during this period (Chapin and Bloom 1976; Mullen et al. 1998; Jaeger et al. 1999; Sturm et al. 2001; Grogan and Jonasson 2003; Forsum et al. 2008). Furthermore, the spring nutrient pulse could strongly influence the spatial patterning of vegetation types and primary production across the landscape since high rates of hydraulic conductivity at this time may result in significant nutrient transfers to lower elevation ecosystems. Alternatively, a portion or all of the nutrients released during spring may be exported to the aquatic system (Whalen and Cornwell 1985; Everett et al. 1996; Michaelson et al. 1998; Jones et al. 2005; Balcarczyk et al. 2009), maintaining ecosystem-wide nutrient limitations on production in vegetation communities (Vitousek et al. 1998).
Spring soil biogeochemical studies in arctic wet sedge meadow and dry heath tundra ecosystems indicate that soil soluble nutrient pools and soil microbial biomass are relatively high during winter and crash rapidly during spring to relatively stable growing season levels (Edwards et al. 2006; Larsen et al. 2007). The sharp drop in soluble nutrient pools may occur shortly before (Edwards et al. 2006) or after (Larsen et al. 2007) the soil microbial biomass declines at the end of snow pack thaw. These findings are also consistent with alpine biogeochemical studies, which report a decline in microbial biomass nitrogen and a flush of plant-available nutrients at thaw (Brooks et al. 1998). A substantial time gap between snowmelt and aboveground plant production in both arctic and alpine tundra suggests that the potential for net nutrient release and export depends on the internal dynamics of nutrient exchange between soil microbes and the soil solution. However, the very rapid microbial response to environmental and substrate fluctuations is difficult to capture in field studies. As a result, there are very few records of soil processes in the winter–spring period at the temporal frequency required to capture these microbial-biogeochemical dynamics, and none in mesic tundra soil.
Thaw dynamics may also be influenced by conditions during the preceding winter. In particular, winter snowpack depth may be an important control on soil biogeochemistry and vegetation community heterogeneity across arctic tundra ecosystems, and snow depth is variable spatially and temporally (Olsson et al. 2003). Climate change may enhance this spatial and temporal variability in both snow melt water inputs and snow nutrient inputs to thawing soils, with the consistently predicted earlier snowpack thaw and an increase in winter precipitation across the Arctic by 5–35% over the next century (ACIA 2005). The depth and nutrient content of the winter snow pack is an important driver of spring hydrology and biogeochemistry in boreal and alpine soils (Bowman 1992; Brooks et al. 1998; Petrone et al. 2007). For instance, the N from the snowpack appears to be retained during spring thaw in arctic tundra and boreal forest (Cooper et al. 1991; Tye et al. 2005; Petrone et al. 2007) and therefore may have a large effect on ecosystem biogeochemical dynamics. In addition, soil saturation may be longer or more intense under deeper snow during the thaw period when drainage is constrained by patches of snow and a shallow active layer, which may increase opportunities for organic matter mobilisation (Hobbie and Chapin 1996; Brooks and Williams 1999; Jones et al. 2005; Petrone et al. 2007). To the best of our knowledge, the potential influences of the snow pack nutrient content or thaw melt-water incubation period on spring soil microbial and biogeochemical dynamics have not yet been characterised for arctic tundra soils.
Deeper snow may also impact soil organic matter during the winter by increasing the mobilization of nutrients from litter and organic matter to more labile pools in the microbial biomass and soil solution. Snow insulates against air temperature effects, at least from a minimum depth of 30-40 cm (Cline 1995) up to 100 cm in depth (Grogan and Jonasson 2006) stabilizing soil temperatures and increasing winter soil temperature minima (Walker et al. 1999; Schimel et al. 2004; Nobrega and Grogan 2007; Buckeridge and Grogan 2008). As a result, soil temperatures appear to be sufficient to promote slow winter soil microbial activity that is cumulatively important due to the length of arctic winters. Experimentally deepened snow exacerbates this effect, significantly increasing C loss and N mobilization in arctic tundra soils (Schimel et al. 2004; Larsen et al. 2007; Nobrega and Grogan 2007; Buckeridge and Grogan 2008). In addition, increasing microbial C limitation to growth and activity under deeper snow is expected as winter progresses, as available substrate from the growing season litter and root exudates are consumed by this enhanced microbial activity (Lipson et al. 2000; Brooks et al. 2004; Buckeridge and Grogan 2008). This substrate limitation to growth and activity, together with rapid changes in soil temperature and osmotic potential at thaw, are theorized to be detrimental to a large proportion of the soil microbial community, leading to death, lyses of the microbial cells, and release of the nutrients stored in the microbial cytoplasm (Morley et al. 1983; Skogland et al. 1988; Schimel et al. 2007). However, although these mechanisms for microbial decline have been documented in the laboratory, field investigations of the environmental and substrate conditions that trigger this microbial decline and nutrient pulses in arctic tundra are necessary to evaluate their overall significance.
To characterize the pattern of spring biogeochemistry in mesic arctic tundra and to quantify the impact of deepened snow on soil solution and microbial C, N and P dynamics, we sampled these nutrient pools every 3 days from winter until spring in ambient and experimentally deepened snow plots. We hypothesized that biogeochemical peaks would be elevated and last longer under deeper snow as a result of larger winter soil microbial and soluble C, N and P pools and a longer thaw period, and not as a result of nutrients that have accumulated in the snow pack. We also hypothesized that discrete biogeochemical phases such as peaks in microbial and soil solution pools could be matched to environmental conditions under the thawing control and deepened snowpack, indicating which factors contribute to microbial turnover and biogeochemical dynamics during the winter–spring transition.
Methods
Site description and experimental design
This study was conducted in the winter and spring of 2007 in a mesic birch hummock ecosystem at the Tundra Ecological Research Station (TERS) at Daring Lake, Northwest Territories, Canada (64°50′ N, 111°38′ W). Daring Lake is located 300 km northeast of Yellowknife, in the Coppermine River watershed, and the hummock ecosystem at this site is located in a valley, midway along a catena that drains into the lake. The soils and vegetation along this moisture gradient have a circumpolar distribution and this particular site has been previously described (Nobrega and Grogan 2007, Nobrega and Grogan 2008, Buckeridge and Grogan 2008, Lafleur and Humphreys 2008). Briefly, the soils are Orthic Dystric Turbic Cryosols (Soil Classification Working Group 1998) and consist of an organic horizon ~3–20 cm deep above cryoturbated silt-sand mineral horizons. The ecosystem is characterized by hummocks 10–30 cm high and deciduous dwarf birch (Betula glandulosa) shrubs that are 10–40 cm tall and attain ~10–30% of the areal coverage of the hummocks, interhummocks (mid-elevation), and hollows. The remaining cover is a mixture of other deciduous shrubs, ericaceous shrubs, sedges, mosses and lichens (Nobrega and Grogan 2008).
Climate records from the Daring Lake weather station (1996–2008; Bob Reid, Department of Indian and Northern Affairs Canada (INAC) Water Resources Division, unpublished data), ~500 m from the study plots, indicate a mean diel minimum air and soil (5 cm depth) temperatures of −38°C and −26°C, respectively, in mid-January; and mean diel maximum air and soil (5 cm depth) temperatures of 15°C and 12°C, respectively, in late July. Maximum snow depth in exposed areas averages 37 cm (range: 15–59 cm) by late winter, followed by a snow melt period averaging 15 d (range: 5–28 d). The snow-free season lasts ~120 d (typically early June to late September) with an average summer rainfall of 141 mm.
A snow experimental treatment was established in 2004 to increase the depth and duration of snow cover within birch hummock tundra vegetation (Nobrega and Grogan 2007). Snow fences (1.2 m tall and 15 m long; n = 5) were erected perpendicular to the prevailing wind and created snowdrifts the length of the fence on either side, which had a mean maximum depth of 1.1 m (2006–2007). These drifts were higher than ambient snow depth for at least 11 m out from each fence on either side, stabilizing and increasing the lowest winter soil diel mean temperature from −18°C to −12°C at 5 cm depth (Buckeridge and Grogan 2008). In addition, the snow fences extended snow cover in the spring by 1–2 weeks. Control sites (unfenced, 15 m long; n = 5) were established parallel to the fences in similar vegetation yet offset from the fences by more than 30 m to ensure clear separation from the snow fence drift areas.
Soil temperature and soil water
Daily mean soil temperature was monitored at 2, 5 and 10 cm depth over the winter and spring in one control plot (n = 2, 4 and 2 probes, at the respective depths) and two deepened snow plots (n = 2, 7 and 1 probes, at the respective depths), using thermocouple probes (in situ precision coefficient of variation 1.7–7.4%) connected to CR 10X dataloggers (Campbell Scientific, Logan, UT). Soil water content was measured gravimetrically on all collected soil sample (total water) from each plot, and as volumetric water (liquid water) in two control plots (n = 2 total) and one deepened snow plot (n = 2 total) with 30 cm long dielectric permittivity probes (Water Content Reflectometer, Campell Scientific) inserted at an angle to measure moisture within the top 10 cm of soil, and connected to the same dataloggers that recorded soil temperature.
Soil sampling protocol
Soil samples were collected in winter, on Julian day 99 from all plots, returned to the lab at Queen’s University by the following day and kept cold (between −10 and 0°C) until soil processing on day 101. This was followed by high-frequency field sampling every 3 days over 16 sample dates, beginning on day 130 and lasting until day 175, the start of bud break for B. glandulosa.
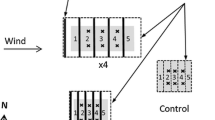
Sequential sampling in all plots progressed from one side of the plot to the other along a line ~1 m then ~2 m north (maximum drift) and parallel to each fence. Control plot sampling location was arbitrary and without preconceived bias. Care was taken to not to displace more of the snow pack than was necessary. At the same time each afternoon, a ~1 × 1 m pit (0.5 × 0.5 m in the control plots) was shoveled down to the vegetation, the snow from each day’s snowpit was laid out with approximate stratigraphy to the west (ontop of the refilled snow from the previous day’s pit). Snow was brushed gently from around the vegetation before removing a block of tundra, and samples were collected by hammering an axe blade down to the bottom of the soil organic layer (average depth 5.3 cm, range 2.9–9 cm) around the edges of each sample. Sample characteristics such as above-ground vegetation were not pre-selected, but determined by the location of the base of the snowpit. As thaw approached and vegetation became exposed, this arbitrary sampling protocol was maintained; therefore our results represent the range of possible ecosystem heterogeneity, including hummocks, interhummocks and hollows. After the sample was removed and soil temperature recorded at the base of the pit, the snowpits were refilled with approximate stratigraphy. Sampling moved 1 m east to the next undisturbed patch on the next sample day. The subnivean environment was exposed for approximately 1 h at the base of the pit (0.25 h in the control plots), and soil temperature (with handheld probes) did not appear to fluctuate in response to this exposure. All samples were stored overnight in plastic bags in a cooler in the field lab, then processed the following morning (i.e. soils were maintained frozen if collected frozen).
Soil handling, in the lab at Queen’s University and in the field, was minimized to restrict disturbance of the soil microbial community and soil biogeochemistry while homogenizing each sample and to prevent thawing the frozen soils during handling instead of during extraction. Above ground vegetation and litter was cut off at the moss green–brown layer, soil was chopped (if frozen) or crumbled (if thawed) into small pieces (maximum diameter ~10 mm), large roots (>2 mm) were removed, and soils were immediately subsampled for biogeochemical or microbial extraction. Each soil sample was: (a) extracted in filter-sterile water (0.2 μm pore-size cellulose membrane, Fisher) to estimate water-extractable organic and inorganic soil solution pools of C, N, and P; (b) extracted in filter-sterile 0.5 M K2SO4 before and after fumigation with chloroform to estimate microbial biomass C, N and P; and (c) oven-dried to estimate gravimetric soil water content (65°C). Chloroform-fumigation direct-extraction (CFE) was for 24 h in a darkened vacuum desiccator jar at ~15°C directly followed by extraction (Brookes et al. 1985). All extract samples were shaken manually several times for a minimum of 1 h in extractant, filtered through a 1.2 μm pore-size glass fiber filter (Fisher G4), then frozen at −20°C until biogeochemical analyses.
Snow sampling
At the end of ‘deep cold’ (Olsson 2003), on day 129, a snow pit was dug in each plot, and snow layer thickness, temperature, location from the base of the snow pack, snow crystal description and estimated relative density were recorded. Aluminium sampling tubes (125 or 250 cm3) were used to remove a snow sample from any layer >2.5 cm thick. These samples were placed in pre-weighed sterile sampling bags and stored in a cooler and returned to the field lab. Sample weight and volume were recorded and samples were frozen and returned to Queen’s University for biogeochemical analysis of dissolved organic carbon (DOC), dissolved total nitrogen (DTN), ammonium-nitrogen (NH4 +–N) and nitrate-nitrogen (NO3 −–N). Snow pack nutrient content on an aerial basis was estimated from the nutrient concentrations, snow density and the depth of each layer. Nutrient contents in unsampled snow layers (average = 11% of the total snow pack depth, range 2.1–23.1%) were estimated based on field observations of relative density and similarity in crystal structure to that of measured layers. Ice layers were unsampled and averaged 3% of the total snowpack depth; ice layer density was assumed to be 0.91 g cm−3. In addition to these initial snow pit measures, snowdepth above each soil sample was measured on every soil sampling occasion.
Biogeochemical analyses of soil extracts and snow
Dissolved NH4 +–N, NO3 −–N in water extracts and snow water, dissolved total phosphorus (DTP) in water extracts, and dissolved phosphate–P (PO4 3−–P) in salt and water extracts were determined colourimetrically, using automated flow analysis (Bran-Leubbe Autoanalyzer III, Norderstadt, Germany) and the salicylate (NH4 +–N; Mulvaney 1996), sulphanilamide (NO3 −–N; Mulvaney 1996), alkaline persulfate (DTP; Lathja et al. 1999) and absorbic acid (PO4 3−–P; Kuo 1996) methods. Dissolved organic C and total N contents in the fumigated and non-fumigated salt extracts and in the water extracts and snow were determined by oxidative combustion and infrared (DOC; Nelson and Sommers 1996) or chemiluminesence (DTN) analysis (TOC-TN autoanalyzer, Shimadzu, Kyoto, Japan). Dissolved organic P (DOP) was calculated as the difference between DTP and PO4 3−–P, and dissolved organic N (DON) was calculated as the difference between DTN and inorganic N (NH4 +–N + NO3 −–N), in water extracts. Microbial biomass C, N and P contents (MBC, MBN and MBP) were calculated as the difference between DOC, DTN or PO4 3−–P in fumigated and non-fumigated salt-extracts. The microbial P calculated as the difference between DTP and DOP in fumigated extracts was not significantly greater than the difference between DTP and PO4 3−–P in fumigated extracts, as for others (Jonasson et al. 1996), so we calculated MBP with the latter. No correction factors for fumigation or PO4 3−–P extraction efficiency were applied. All C, N and P concentrations in the soil extracts were corrected for the dilution associated with the moisture content of each soil sample.
Statistical analyses
Effects of the deepened snow treatment on environmental and biogeochemical variables over the 17 dates were tested with separate repeated measures analyses of variance (RM ANOVA), with plot (n = 5) nested in treatment as a random effect and with treatment and time (sample date) and their interaction as fixed effects. The statistical significance of individual days, and interactions, were assessed with Tukey’s HSD post-hoc analyses. These analyses were performed with JMP 7.0 (SAS 2007, Cary, NC). Data were transformed where necessary to meet requirements of normality and homogeneity of variance. Figures show untransformed means and all significant results are reported.
The structure within the biogeochemical and environmental dataset over time and between treatment and control plots was examined by ordinating the data using non-metric dimensional scaling (NMS; Kruskal 1964). We separated the data into 4 time phases on the basis of past climate summaries for this research area (Bob Reid, unpublished data), and after exploratory cluster analyses of the data matrix. These phase distinctions were tested by contrasting the data in each phase using multi-response permutation procedure (MRPP; Mielke 1991). The NMS, cluster analysis and the MRPP were all performed with PC-ORD 5.0 software (McCune and Mefford 2006). NMS is an iterative multivariate ordination technique that is suited to non-linear data that are not normally distributed (McCune and Grace 2002). Data within each variable were log-transformed where necessary, and relativized to the maximum within each variable, to improve the effectiveness of the distance measure (McCune and Grace 2002). The NMS provided a series of solutions, stepping down in dimensionality from 6 to 1, using a Sørensen distance measure and a maximum of 500 iterations in 50 runs with real data to find the best positions for all samples along the ideal number of dimensions, beyond which additional dimensions provide only small reductions in stress. Statistical significances of axes were assessed by comparing observed final stress with the final stress in 50 runs of randomized data using a Monte Carlo test (McCune and Grace 2002). The NMS matrix included five environmental variables (soil and air temperature, soil gravimetric and volumetric water and snow depth) and 10 biogeochemical variables (soil solution DIN, dissolved PO4 3−–P, DOC, DON, DOP and MBC, MBN, MBP, MBC/N and MBC/P). Each data cell within the matrix was a mean of the spatial replicates, as three of the environmental data variables (air and soil temperature and soil volumetric water), although collected within the sample plot area, were not collected at the same location as the soil samples. We used Pearson correlations to test for significant associations between variables and the axes scores. The cluster analysis used a Euclidean distance measure and Ward’s linkage measure to create a dendogram that indicated phase and treatment separation. The MRPP yielded a P-value to evaluate how likely it is that the observed difference between groups (treatment or phase) was due to chance, as well as the chance-corrected within-group agreement (A), which described within-group homogeneity compared to random expectation (McCune and Grace 2002). This method is not adversely affected by the heterogeneity of variance common to different group sample sizes (McCune and Grace 2002). ‘A’ values range from 1 (when all items within a group are identical) to 0 (when heterogeneity within groups equals expectation by chance).
Results
Winter–spring temperature and soil water dynamics
Daily mean air temperature during the study period generally increased from a low of −15.9°C on Julian day 107 to a maximum of +14.9°C on day 175, but fluctuated by as much as 6°K d−1 (Fig. 1a). Snow depth decreased from a mean peak of 31 cm on day 130 to complete thaw by day 151 in the control plots, and from a mean peak of 101 cm on day 136 to complete thaw by day 166 in the deepened snow plots. Therefore, the snow was significantly deeper (F 1,169 = 240, P < 0.0001), complete melt was 15 days later, and the rate of thaw was more consistent and rapid (Treatment × Time: F 16,169 = 40, P < 0.0001) in the snowfence plots (Fig. 1b).
Daily mean (black line), maximum and minimum air temperatures (a); mean snow depth (cm, n = 5 each) (b); soil temperature at 2 (grey line), 5 (black line with closed or open circles) and 10 (grey dashed line) cm depth (oC; n = 2, 4, and 2 for respective control plot soil depths and n = 2, 7, and 1 for respective snow fence soil depths) (c); soil volumetric water (%; n = 2) (d); and soil gravimetric water (i.e. liquid and frozen g H2O/g dw soil; n = 5) (e) in control and deepened snow (snow fence) plots in birch hummock vegetation at Daring Lake, NWT during the winter–spring of 2007. Error bars indicate ± one standard error. Vertical lines distinguish the environmental and biogeochemical phases described in the text
Daily mean soil temperatures at 2, 5 and 10 cm depth in both control and snowfence plots all increased in a similar step-wise fashion, hovering near −10°C from day 99 to day 124 and increasing and staying near −5°C until day 145. Soil temperatures then increased to 0°C on day 145 after a step-like climb over 5 days in the control plots, or to just below zero (−0.65°C) on day 146 after a rapid climb over 2 days in the snowfence plots (Fig. 1c). Daily soil temperatures were very steady between steps in the deepened snow plots, and were much more dynamic in the control snow plots, with peaks and drops closely tracking air temperature patterns (Fig. 1a, c). Daily minimum soil temperatures at 10 cm depth in the control plots rose above zero by day 151, whereas snowfence soils remained just below zero at 10 cm depth until the end of the study period on day 175 (Fig. 1c). Hourly temperature data were not recorded at our sites, but hourly data from a nearby site (500 m) in a similar vegetation type indicated no soil freeze–thaw cycles during this spring thaw (Bob Reid, INAC Water Resources Division, unpublished data).
Soil liquid water contents (as indicated by dielectric probe data) increased slightly on day 125 in control plots, and increased substantially in both the treatment and control soils after day 145, with a slower increase under deepened snow (Fig. 1d). Gravimetric soil moisture (frozen plus liquid water) remained static in both ambient and deepened snow plots until day 145 when mean diel air temperatures rose substantially above 0°C (Fig. 1a, e). Melting of the snow pack thaw did not noticeably increase overall (gravimetric) soil moisture in control plots, suggesting that either ground surface runoff or sublimation and evaporation from the snow pack were high, and that increases in volumetric (liquid) water were primarily a result of melting soil ice. By contrast, snow melt from the deepened snow pack raised the gravimetric soil water content considerably during snow thaw, and significantly on days 160 and 163 (Fig. 1d), resulting in significant treatment, time, and treatment by time interaction effects (Treatment, F 1,169 = 5.8, P = 0.042; Time, F 16,169 = 4.9, P < 0.0001; Treatment × Time, F 16,169 = 4.0, P < 0.0001).
The control and deepened snow pack contained 117 and 370 l snow water m−2, respectively, at the start of spring snowmelt (day 129). Correspondingly, control and deepened snow plot soil water (computed to the base of the organic layer) increased from 43 and 32 l soil water m−2 at the start of the spring study period to their wettest points during spring thaw at 46 (day 145) and 94 (day 160) l soil water m−2, respectively. Soils at the base of the deepened snow pack were noticeably saturated, and occasionally submerged under several centimetres of water from the end of May until early June (day 147–163). This large soil water contents in deepened snow plots during that time buffered soil temperature against rising and falling temperatures, maintaining the soils at or just below 0°C (Fig. 1c) and delaying the increase in peak volumetric (liquid) water by almost 2 weeks as compared to control plots (Fig. 1d). Together these dynamics in air and soil temperatures, snow melt and soil water indicate four discrete phases in soil environmental conditions under ambient snow: Winter (soils cold, with very little liquid water); Early thaw (soils warmer but still frozen and with very little liquid water); Late thaw (soils at temperatures close to 0°C with substantial liquid water); and Spring (soils at temperatures above 0°C with substantial liquid water) (Fig. 1a–e). The transitions between phases were simultaneous in soils under deepened snow although the magnitudes of the soil liquid water increase in late thaw were much greater.
Snowpack structure and nutrients
Temperatures throughout the deepened snow pack were relatively cool (−3 to −4°C) on the afternoon of day 129 when the snowpits were studied (data not shown). By contrast, the ambient snowpack profile was relatively warm (just below 0°C) from the base up to close to the surface, with markedly cooler temperatures right at the surface that matched simultaneous air temperatures (~ −4°C). The deepened snow pack was about 3 times higher (t 1,9 = 13, P < 0.0001) and had a correspondingly larger snow water equivalent (SWE), 3.5× that of the control plot snowpacks (t 1,9 = 13, P < 0.0001).
NO3 −–N pools in the snowpack of control plots (4.4 mg m−2 (S.E. ± 1.2)) were similar to the nitrate content of the soil organic layer (0–10 mg m−2). By contrast, there were relatively small amounts of DOC (11 (±3.1) and 120 (±59) mg m−2), DTN (2.6 (±1.2) and 25 (±7.4) mg m−2) and NH4 +–N (1.0 (±0.67) and 2.5 (±0.55) mg m−2) in the snow packs (control and deepened snow, respectively) compared to the soil solution (see ahead). The snow packs did not differ significantly in snow density or nutrient concentrations, but the deepened snow held larger water and nutrient pools; especially NO3 −–N (33 (± 3.6) mg m−2; t 1,9 = 7.4, P < 0.0001). NO3 −–N and NH4 +–N were distributed evenly throughout the snow pack, whereas DOC and DTN concentrations were largest near the base of the snow pack, but detectable at low concentrations throughout (data not shown).
Soil solution inorganic and organic nutrients
Soil solution inorganic nutrients tended to be low in winter, variable during the thaw phases and return to low values in the spring, whereas organic nutrients were relatively high in the winter, variable during thaw, and then also dropped to very low values in the spring. The soil DIN content was dominated by NH4 +–N on all sampling dates, with very low NO3 −–N contents (ranging from 0 to 1.5 μg g−1dw soil), except for a NO3 −–N spike (12.7 μg g−1) in one deepened snow plot on day 154. DIN content was 10–15× higher in deepened snow plots compared to controls during the late thaw phase and was significantly higher on day 157 (Fig. 2a), resulting in significant overall effects of treatment, sampling date and their interaction (Treatment, F 1,169 = 7.0, P = 0.030; Time, F 16,169 = 11, P < 0.0001; Treatment × Time, F 16,169 = 2.7, P = 0.0013). Soil dissolved PO4 3−–P contents fluctuated dynamically and differently in the control and deepened snow plots in the early thaw period (Fig. 2b) resulting in a significant sampling date effect (F 16,169 = 7.2, P < 0.0001) and treatment by sampling date interaction (F 16,169 = 2.3, P = 0.005), but there was no overall treatment effect on PO4 3−–P across the study.
Dissolved inorganic nitrogen (NH4 +–N plus NO3 −–N) (a) and phosphorus (PO4 3−–P) (b) contents in the water-extracted soil solution of birch hummock tundra during the winter–spring of 2007 under ambient (control) and deepened snow. Error bars indicate ± one standard error. Vertical lines distinguish the environmental and biogeochemical phases described in the text
DOC contents in both control and deepened snow plots were elevated in late winter snow pack (day 139) as compared to the earlier deep winter (day 99) and later thaw and spring sampling dates (Time, F 16,169 = 44, P < 0.0001; Fig. 3a). DOC contents fluctuated in the early thaw phase and then declined as the soil liquid water levels increased in both the ambient and deepened snow plots. However, DOC content increased significantly under deepened snow relative to controls on days 157–163, when soil water also peaked (Treatment × Time, F 16,169 = 6.1, P < 0.0001; Figs. 1d, e, 3a).
Dissolved organic carbon (a), nitrogen (b), and phosphorus (c) contents in the water-extracted soil solution of birch hummock tundra during the winter–spring of 2007 under ambient (control) and deepened snow. Error bars indicate ± one standard error. Vertical lines distinguish the environmental and biogeochemical phases described in the text
DON contents were high in both deep winter and under the late winter snowpack, regardless of snow depth, and like DOC dropped to low levels when soil liquid water increased (Fig. 3b). As with DOC, DON increased under the melting deepened snow pack so that there were significant sampling time and treatment by time interaction effects (F 16,169 = 29, P < 0.0001 and F 16,169 = 1.8, P = 0.041, respectively), although high spatial variability in DON pools resulted in the treatment difference being insignificant overall (Fig. 3b).
DOP contents were dynamic and spatially variable in late winter relative to deep winter or spring (Fig. 3c). As with DOC and DON, DOP contents were elevated in the deepened snow plots under the melting snow pack (i.e. late thaw phase), leading to significant sampling time and treatment by sampling time effects (F 16,169 = 8.1, P < 0.0001 and F 16,169 = 2.2, P = 0.0075, respectively; Fig. 3c).
Soil microbial biomass C, N and P
Soil microbial C content was elevated in deep winter and had generally dropped by the start of the early thaw phase when soils climbed to −5°C, regardless of snow depth (Fig. 4a). MBC contents in control and deepened snow plots tended to increase during the early thaw phase and then dropped sharply and remained low in control plots (Fig. 4a). By contrast, MBC contents were significantly higher in the deepened snow plots overall (F 1,169 = 21, P = 0.0017), and increased in the second half of the late thaw to a significantly higher peak on day 160 as compared to other sampling dates (Treatment × Time, F 16,169 = 3.0, P = 0.0003), that corresponded with the peak in gravimetric water (Fig. 1e). MBC under the rapidly melting deep snow plots decreased sharply over the next 6 days as the snow completely melted and snowfence soil temperatures rose above zero. Afterwards, MBC was similar in control and snowfence plots and was at its lowest level in the last three sample dates of the study (Time, F 16,169 = 10, P < 0.0001; Fig. 4a).
Soil microbial biomass carbon (a), nitrogen (b), and phosphorus (c) contents in birch hummock tundra during the winter–spring of 2007 under ambient (control) and deepened snow. Error bars indicate ± one standard error. Vertical lines distinguish the environmental and biogeochemical phases described in the text
MBN and P contents in the snowfence and control generally matched each other and the pattern for MBC in the early thaw phase (Fig. 4a–c). MBN and MBP contents in control plots were also similar to MBC in the late thaw phase. By contrast, MBN and MBP contents in the deepened snow plots increased rapidly as soon as liquid water increased at the onset of late thaw, but MBC did not rise until later. The treatment by time effect was significant for MBP, with significantly higher P accumulation in the microbial biomass under deepened snow on days 157 and 160 (F 16,169 = 3.0, P = 0.0003). In addition, the MBN and MBP increases occurred at least 9 days before peak MBC in the same plots, suggesting microbial accumulation of nutrients occurred well before microbial growth enhancement. The declines in MBC, MBN and MBP were all synchronous and corresponded exactly with decreasing soil moisture contents and a return to ambient unsaturated soil conditions at the end of snowmelt. Both MBN and MBP were significantly higher in deepened snow plots overall (MBN, F 1,169 = 29, P = 0.0007; MBP, F 1,169 = 14, P = 0.0056) and both control and deepened snow plots MBN and MBP varied significantly with sampling date (MBN, F 16,169 = 2.9, P = 0.0004; MBP, F 16,169 = 14, P < 0.0001).
Microbial N and P accumulation were similar between treatments but fluctuated from N and P accumulation (i.e. low MBC/N or MBC/P) to MB growth (i.e. high MBC/N or MBC/P; Fig. 5a, b), leading to a significant time of sampling effect for all the measured microbial biomass ratios (MBC/N, F 16,169 = 8.8, P < 0.0001; MBC/P, F 16,169 = 2.6, P = 0.0014; MBN/P, F 16,169 = 14, P < 0.0001). The late thaw phase was marked by excess MBP in the deepened snow plots (i.e. low MBC/P and MBN/P; Fig. 5b, c) and increasing microbial P shortages (i.e. high MBC/P and MBN/P) in control plots, as well as increasing microbial N shortages (i.e. high MBC/N) in both control and deepened snow plots (Fig. 5a–c). MBC/N dropped sharply in both control and deepened snow plots in spring, suggesting N availability was high relative to C. Very high spatial variation in MBC/P and MBN/P in spring suggests that P availability was very low and rapidly immobilized at this time, and is supported by low inorganic and organic P soil solution contents at this time (Figs. 2b, 3c, 5b, c).
Soil microbial biomass carbon/nitrogen (a), carbon/phosphorus (b), and nitrogen/phosphorus (c) ratios in birch hummock tundra during the winter–spring of 2007 under ambient (control) and deepened snow. Error bars indicate ± one standard error. Vertical lines distinguish the environmental and biogeochemical phases described in the text
Winter–spring phases
The NMS ordination tested for structure in the environmental and soil and microbial biogeochemistry dataset (Fig. 6), while the MRPP tested for significant effects of the treatment and significant clustering among the data. The NMS solution had 2 dimensions, with a low final stress of 9.3 after 73 iterations (Fig. 6). The data from the control and deepened snow plots showed similar patterns through the winter–spring transition, however, the analysis clearly indicated a significant treatment effect (A = 0.09, P = 0.0036). In particular, the largest shift along the first axis occurs at complete snowmelt, which is at the beginning of late thaw in control plots and at the beginning of spring in the snowfence plots (Fig. 6). The first axis explains most of the variation in the data (84.2%) and was strongly and positively correlated with soil temperature, VWC (r 2 = 0.72), and air temperature (r 2 = 0.58), and negatively correlated with DOC (r 2 = 0.78) snow depth (r 2 = 0.78), MBP (r 2 = 0.62), DOP (r 2 = 0.62), PO4 3−–P (r 2 = 0.62), and DON (r 2 = 0.59). The snowfence plots were generally lower on the second axis (Fig. 6). The second axis explains 11.3% of the variation in the data and is strongly negatively correlated with snow depth (r 2 = 0.73), MBC (r 2 = 0.71), PO4 3−–P (r 2 = 0.70), MBP (r 2 = 0.67), DOP (r 2 = 0.59), GWC (r 2 = 0.56), MBN (r 2 = 0.55), DOC (r 2 = 0.54), DON (r 2 = 0.51) and positively correlated with MBC/P (r 2 = 0.51).
Ordination of environmental and biogeochemical data collected in winter, then every 3 days throughout the early thaw-spring transition in birch hummock tundra. The first axis is aligned with soil temperature (r 2 = 0.78). The data points represent non-metric multidimensional scaling (NMS) scores for the samples means (n = 5 spatial replicates) from a matrix of 5 environmental, 5 microbial and 5 substrate variables measured in control and deepened snow plots. The grey lines connect the time sequences of measurements from the sample plots. ‘Phases’ represent significantly different (MRPP) environmental and biogeochemical time periods of thaw in this low arctic tundra ecosystem; ‘complete snowmelt’ indicates the date on which all snow disappeared from the control and deepened snow plots
Cluster analysis of the biogeochemical and environmental data indicated that the four phases suggested by visual inspection of the environmental data (Fig. 1a–e) were indeed discrete, and furthermore that the control and snowfence plots differed from each other during the late thaw phase (data not shown). The strongest MRPP model supported these five clusters (A = 0.52, P < 0.0001), all of which were significantly different from each other in separate pairwise comparisons (MRPP; A = 0.15–0.53; P < 0.008): 1. ‘Winter’ (day 99, n = 2); 2. ‘Early thaw’ (days 130–145; n = 12); 3. ‘Late thaw-control’ (days 147–163; n = 6); 4. ‘Late thaw-deepened snow’ (days 147–160; n = 6); and 5. ‘Spring’ (days 166–175; n = 8) (Fig. 6).
Discussion
Environmental controls on soil biogeochemistry and the phases of thaw
Our high frequency measurements of environmental and biogeochemical variables during the winter–spring transition allowed us to test for significant patterns, structure and inter-relationships within the integrated dataset. The results are conclusive (for this year at this site at least): the winter–spring transition can be considered as consisting of four distinct phases (winter, early thaw, late thaw and spring) representing discrete and corresponding patterns in environmental conditions and biogeochemical pools. This characterisation should provide a useful template for future process-based studies of soil thaw in the low arctic and possibly in other seasonally snow-covered ecosystems. Our integration of biogeochemical and environmental patterns into these phases builds on previous broader temporal scale environmental characterisations of the full cold season in the Alaskan Arctic (Olsson et al. 2003). The early and late thaw phases reported here are refinements of the ‘thaw’ phase characterized by those authors. Furthermore, our analyses indicate that landscape variation in snowdepth is a significant factor influencing biogeochemical dynamics at this time. The winter–spring phases outlined here appear to match high frequency biogeochemical and environmental patterns in sedge meadow, heath and birch forest understory sites during this time of year (Edwards et al. 2006; Larsen et al. 2007), suggesting that this pattern may be widespread in wet, mesic and relatively dry ecosystems across the Low Arctic.
This study indicates that soil biogeochemical pools and microbial immobilization of C, N and P are very dynamic between −5°C and just below 0°C, but that these biogeochemical dynamics stop and microbial biomass declines simultaneously, as soon as the daily mean soil temperature rises above 0°C. This pattern is consistent under both ambient and deepened snow because the soil temperature threshold occurs as soon as snowmelt is complete and regardless of differences between ambient and deepened snow soil gravimetric and volumetric water contents. For instance, during the 15 days of snowmelt in the control plots, soil water was relatively low and still mostly frozen; during the 20 days of snowmelt in the deepened snow plots, total soil water (ice plus liquid) was relatively high and thawing resulted in a steady accumulation of liquid water. Nevertheless, the peaks in soil solution nutrients and microbial biomass C, N and P ended exactly when snowmelt was completed and soil temperature crossed 0°C in both cases. Although not presented as such in other studies, this microbial decline with the end of snowmelt is consistent in alpine moist meadow tundra, arctic wet sedge tundra and arctic dry heath tundra (Brooks et al. 1998; Edwards et al. 2006; Larsen et al. 2007). These results suggest that the period of sub-zero soil temperatures is the principal phase of soil microbial and biogeochemical dynamics during winter–spring transitions in these ecosystems. We suggest that, on a microbial scale, microsite variation in liquid soil film thickness, local soil water phase changes and possibly fluctuating reduction–oxidation potentials below 0°C are critical drivers of thaw biogeochemistry (Sturm et al. 2005). Snow melt water infiltration of soil pore spaces may expose previously dormant fungi and bacteria to thaw and fresh substrate, possibly promoting specialized substrate use by particular microbial functional groups (Polyanskaya et al. 2008). Soil water inputs may also facilitate trophic level interactions, an important control over biogeochemistry in soil systems (Wardle 2002). For instance, soil water phase changes may promote macrofaunal hatching (Sulkava and Huhta 2003) and liquid water connections between soil pores may promote predation, possibly promoting microbial decline.
Our data bring into question a number of other possible mechanisms for microbial decline that have been previously proposed. First, soil freeze–thaw cycles have been proposed as a cause for decline in some microbial species and thus for biogeochemical flushes at spring-thaw. Although supported by several laboratory studies (Schimel and Clein 1996; Herrmann and Witter 2002; Larsen et al. 2002; Six et al. 2004; Walker et al. 2006), mesocosm studies using moderate freeze–thaw amplitudes that are more characteristic of arctic tundra field conditions (Grogan et al. 2004), and a field study just after snow melt in Daring Lake (Buckeridge et al. in press), both suggest that diurnal or weather-front freeze–thaw cycles have minor or no effects on spring soil biogeochemistry. In any event, the microbial decline in our study clearly was not associated with high amplitude temperature cycling. Second, osmotic stress has been proposed as a driver of microbial decline (Schimel et al. 2007), and is supported by laboratory studies demonstrating microbial production of osmolytes as a mechanism for bacteria and fungi to deal with various types of stress (Yancey 2005). Osmotic stress may occur during extracellular freezing in early and mid- winter when water is gradually removed from cells by osmosis during thinning of soil water films, and as water vapour transfer into the overlying snow increases concentrations of soil water ions. Osmotic stress may also occur during thaw, when those microbes that have adapted to winter dryness through osmolyte production are suddenly introduced to a burst of fresh water, leading to cell rupture and cytoplasmic release of as much as 10–30% of the microbial C and N (Schimel et al. 2007). However, if this were the principal mechanism of microbial biomass decline, we would expect that soil solution peaks would either lag declines in microbial peaks of C, N and P indicating cytoplasmic loss to the soil solution, or precede MBC, N and P declines as a result of enhanced microbial growth due to the increase in soil solution nutrients; however, declines in all peaks were simultaneous. Third, labile carbon limitation of the winter microbial biomass has been observed in Daring Lake (Buckeridge and Grogan 2008), and has been associated with microbial decline under deep snowpacks in alpine tundra soils (Brooks and Williams 1999; Lipson et al. 2000). However, the soil solution DOC in this study was relatively high during late winter and especially during thaw, and collapsed simultaneously with microbial declines, but not before. Unless these thaw DOC pulses are very low quality and therefore essentially unavailable for soil microbes, the C patterns we observed over the winter–spring transition suggest that although soil microbes may be limited by labile C availability in late winter (Buckeridge and Grogan 2008), this C limitation ends with enhanced DOC pulses in both early and late thaw. Therefore, we suggest that C limitation does not contribute to the microbial and nutrient decline in low arctic tundra at thaw. Further research is required to understand soil biogeochemical and microbial and macrofaunal physiological responses to frozen and liquid water fluctuations below 0°C.
The impact of deeper snow
We observed dynamic fluctuations in inorganic, organic and microbial C, N and P pools under the thawing snow pack that were enhanced in magnitude and duration under deeper snow. Excess soil water inputs in the deepened snow plots may have promoted anaerobic conditions and an anaerobic microbial activity response that is reflected in the larger biogeochemical pulses under deepened snow. However, it is unclear if anaerobosis produced by saturation in the deep snow treatment differs from anaerobosis produced by gas diffusion limitations in frozen soil (Miller et al. 2007), that may have been present under both deep and ambient snow. Deepened snow also enhanced microbial growth (increased MBC) and microbial nutrient accumulation (decreases in MBC/N and MBC/P associated with high MBN and MBP) at sub-zero temperatures. In general, these results are consistent with other studies in arctic and alpine tundra (Lipson et al. 1999; Schimel et al. 2004; Edwards et al. 2006; Larsen et al. 2007). In addition, the strong treatment effect in this investigation indicates that this biogeochemical pattern at thaw is sensitive to winter environmental conditions, as with alpine tundra (Brooks et al. 1998). In particular, the nutrient and microbial peaks under the melting deep snow pack were responsible for creating an enhanced ‘late thaw’ phase of spring biogeochemistry that was associated with significantly larger nutrient mobilization through the soil solution and the microbial biomass.
The early and late thaw phases may be the most sensitive of all winter stages to global change, as a result of ecosystem sensitivity to thaw timing and snowdepth. Climate change predictions consistently indicate that the arctic winter will experience warmer winter air temperatures and increased winter precipitation as a result of warmer air transferring larger amounts of moisture towards the poles (ACIA 2005). In addition, landscape and interannual variability in snowdepth may have large impacts on the timing of thaw (Olsson et al. 2003). At Daring Lake for instance, snow accumulates over the 9 months of winter, peaking in late winter, with interannual variability in maximum snow depth ranging from 15 to 54 cm at one location within this low arctic tundra valley and snow depth variability of 0–100 cm snow depth within the same valley (1996–2008, B. Reid, unpublished data). Therefore, the phases that we have characterized in this study include the natural and future variation in snow depth controls on ecosystem biogeochemistry at thaw. Further sampling in additional years may tell us how common deep snow years are, and/or if the dynamics recorded in this study are consistent with naturally occurring areas of deep snow accumulation across the landscape.
Despite the larger flush of nutrients in the soil solution under the deepened snowpack, we found little evidence that these nutrients are from the snowpack itself. Only nitrate–N was significantly larger than in the ambient snowpack, and large relative to soil solution contents of nitrate. In the saturated thaw soils, anaerobosis may have promoted denitrification, or semi-frozen soils may have favoured overland flow of meltwater and N loss as leachate (Brooks et al. 1999). Earlier studies at this site indicate that denitrification is low in early spring, but primarily as a result of substrate limitation (Buckeridge et al. in press), and our results here indicate that inorganic N and organic C pools are substantial under the thawing snowpack. Snowpack nutrients are retained in Swedish forest and wetland soils (Petrone et al. 2007) and in Alaskan soils on discontinuous permafrost (Jones et al. 2005), but in both of these studies biogeochemical processes at snowmelt promote streamwater N exports from ecosystems that are larger than predicted from snowpack inputs. Our results suggest that a similar pattern of N export may be present in these low arctic mesic tundra soils underlain by continuous permafrost, and that the large microbial mobilization of C, N and P followed by a decline of the soil microbial biomass may be the biotic process responsible for this net N loss at thaw.
Seasonal dynamics of C, N and P
The soil microbial biomass represents a large and labile pool of nutrients in this ecosystem. The microbial pools of C and P were 2–10× larger than the corresponding soil solution pools, while microbial N was 8–40× larger than the DTN pool. In general, these microbial and soil solution pools were larger in the winter–spring transition at this site than in spring, summer or fall (Nobrega and Grogan 2008; Buckeridge et al. in press; Chu and Grogan, in press). We have not measured biogeochemical dynamics at this temporal frequency at other times of year at this site, and cannot conclude that nutrient pulsing does not occur. High frequency seasonal biogeochemical sampling in Alaska indicate that large peaks and crashes in soil solution nutrients occur during the summer in tussock soils, at a similar magnitude to the peaks in NH4 +–N and DOC that we recorded in our hummock soils in spring, although the DON peaks in Alaska were 5× larger (Weintraub and Schimel 2005). Our PO4 3−–P and DOP peaks were high during thaw and dropped to very low levels after snowmelt. Phosphorus soil and microbial pool sizes at thaw were strongly negatively correlated with the main NMS axis in this study, and spring thaw PO4 3−–P peaks were generally higher than have been recorded at other sites, although MBP was similar (Edwards et al. 2006). PO4 3−–P levels in the early half of summer were similarly very low in Alaskan tussock tundra, but peaked in the growing season at 10× our spring soil solution content (Weintraub and Schimel 2005). Therefore, despite environmental and some biogeochemical similarities with other arctic tundra systems that imply a similar pattern of thaw, further seasonal sampling and site comparisons are required before drawing general conclusions about annual patterns of biogeochemical dynamics across the arctic tundra.
Landscape implications of the thaw nutrient flush
The delay in the date of complete snowmelt between control and deepened snow was 20 days, reducing the 1 month period between thaw biogeochemical flushes and bud break of the dominant deciduous shrub B. glandulosa by 2/3rds. Alpine tundra studies have found that the microbial decline at spring thaw produces a flush of nutrients that is taken up by plants in early spring (Brooks et al. 1998; Bardgett et al. 2005), and that certain plant species preferentially acquire this early N flush (Mullen et al. 1998; Jaeger et al. 1999). Nutrient availability, especially N and P, limits plant growth in low arctic tundra (Shaver and Chapin 1980), and early P uptake has been found in some graminoid species, along with an increased N demand (Chapin 1983). The 1 month separation between the arctic tundra nutrient flush and spring deciduous plant growth, and the strong drawdown in nutrients during the spring suggests that at least some of the principal deciduous arctic tundra plant species may not benefit from this thaw flush (Bilbrough et al. 2000). Instead, the deciduous arctic plants may depend on summer and fall nutrient acquisition through mycorrhizal associations, as well as rhizome storage of nutrients over winter and translocation to roots and shoots to begin new growth in spring. However, areas with natural deep snow may have improved synchronicity between spring nutrient flushes and plant uptake of nutrients in the early growing season. Long-term snow fence studies indicate that deepened snow alters vegetation communities (Wahren et al. 2005) and may favour those plant species that can acquire N and P during thaw.
Conclusions
We found large pulses of soil solution and microbial biomass C, N and P under deepened snow and much smaller but still substantial pulses under ambient snow during the winter–spring transition when soils climbed from −5 to 0°C. These results indicate that thaw biogeochemistry is controlled by winter conditions and may be sensitive to present and future variation in snowdepth, soil water and soil temperature. Microbial and soil biogeochemistry were strongly correlated with snow depth, soil water and soil temperature and were separated into distinct phases. Despite this strong correlation, microbial and soil solution nutrient crashes were simultaneous and occurred at the end of snowmelt, regardless of differences in soil temperature and water content between treatment and control plots. We have summarized several possible mechanisms for this microbial decline at thaw, all of which may result in a large direct or indirect source of soil C, N and P as respiration, denitrification, or as leachate to lower elevation systems. This nutrient pulse occurs when soils are still frozen, 1 month before deciduous plant leafout, and thus may not be available to plants in the same ecosystem.
References
ACIA (2005) Arctic climate impact assessment. Cambridge University Press, Cambridge, p 1042
Balcarczyk KL, Jones JB, Jaffe R et al (2009) Stream dissolved organic matter bioavailability and composition in watersheds underlain with discontinuous permafrost. Biogeochem 94:255–270
Bardgett RD, Bowman WD, Kaufmann R et al (2005) A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20:634–641
Bilbrough CJ, Welker JM, Bowman WD (2000) Early spring nitrogen uptake by snow-covered plants: a comparison of arctic and alpine plant function under the snowpack. Arct Antarct Alp Res 32:404–411
Bowman WD (1992) Inputs and storage of nitrogen in winter snowpack in an alpine ecosystem. Arct Alp Res 24:211–215
Brookes PC, Landman A, Pruden G et al (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Brooks PD, Williams MW (1999) Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrol Process 13:2177–2190
Brooks PD, Williams MW, Schmidt SK (1998) Inorganic nitrogen and microbial biomass dynamics before and during spring snowmelt. Biogeochemistry 43:1–15
Brooks PD, Campbell DH, Tonnessen KA et al (1999) Natural variability in N export from headwater catchments: snow cover controls on ecosystem N retention. Hydrol Process 13:2191–2201
Brooks PD, McKnight D, Elder K (2004) Carbon limitation of soil respiration under winter snowpacks: potential feedbacks between growing season and winter carbon fluxes. Glob Chang Biol 11:231–238
Buckeridge KM, Grogan P (2008) Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl Soil Ecol 39:210–222
Buckeridge KM, Cen Y-P, Layzell DB et al (in press) Early spring responses of arctic hummock tundra to freeze–thaw air temperatures, added nitrogen and deepened snow. Biogeochem. doi:10.1007/s10533-009-9396-7
Buckeridge KM, Zufelt E, Chu H et al (in press) Soil nitrogen cycling rates in low arctic shrub tundra are enhanced by litter feedbacks. Plant Soil. doi:10.1007/s11104-009-0214-8
Chapin FS (1983) Direct and indirect effects of temperature on arctic plants. Polar Biol 2:47–52
Chapin FS III, Bloom A (1976) Phosphate absorption: adaptation of tundra graminoids to low temperature, low-phosphorus environment. Polar Biol 2:37–52
Chapin FS, Barsdate RJ, Barel D (1978) Phosphorus cycling in Alaskan coastal tundra—hypothesis for the regulation of nutrient cycling. Oikos 31:189–199
Chu H, Grogan P (in press) Soil microbial biomass, nutrient availability and nitrogen mineralization potential among vegetation-types in a low arctic tundra landscape. Plant Soil. doi:10.1007/s11104-009-0167-y
Cline D (1995) Snow surface energy exchanges and snowmelt at a continental alpine site. In: Tonnessen K, Williams M, Tranter M (eds) Biogeochemistry of snow-covered catchments. International Association of Hydrological Sciences, Wallingford, UK
Cooper LW, Olsen CR, Solomon DK et al (1991) Stable isotopes of oxygen and natural and fallout radionuclides used for tracing runoff during snowmelt in an arctic watershed. Water Resour Res 27:2171–2179
Edwards KA, McCulloch J, Kershaw GP et al (2006) Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biol Biochem 38:2843–2851
Everett KR, Kane DL, Hinzman LD (1996) Surface water chemistry and hydrology of a small arctic drainage basin. In: Reynolds J, Tenhunen J (eds) Landscape function: implications for ecosystem response to disturbance. A case study in arctic tundra. Springer-Verlag, New York
Forsum A, Laudon H, Nordin A (2008) Nitrogen uptake by Hylocomium splendens during snowmelt in a boreal forest. Ecoscience 15:315–319
Grogan P, Jonasson S (2003) Controls on annual nitrogen cycling in the understory of a subarctic birch forest. Ecology 84:202–218
Grogan P, Jonasson S (2006) Ecosystem CO2 production during winter in a Swedish subarctic region: the relative importance of climate and vegetation type. Glob Change Biol 12:1479–1495
Grogan P, Michelsen A, Ambus P et al (2004) Freeze–thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol Biochem 36:641–654
Herrmann A, Witter E (2002) Sources of C and N contributing to the flush in mineralization upon freeze–thaw cycles in soils. Soil Biol Biochem 34:1495–1505
Hobbie SE, Chapin FS (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochem 35:327–338
Jaeger CH III, Monson RK, Fisk MC et al (1999) Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80:1883–1891
Jonasson S, Michelsen A, Schmidt IK et al (1996) Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar: implications for plant nutrient uptake. Oecologia 106:507–515
Jones JB, Petrone KC, Finlay JC et al (2005) Nitrogen loss from watersheds of interior Alaska underlain with discontinuous permafrost. Geophys Res Lett 32:L02401. doi:10.1029/2004GL021734
Kruskal JB (1964) Multidimensional-scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27
Kuo S (1996) Phosphorus. In: Sparks DL (ed) Methods of soil analysis. Part 3, chemical methods. Soil Science Society of America and American Society of Agronomy, Madison, WI
Lafleur PM, Humphreys ER (2008) Spring warming and carbon dioxide exchange over low Arctic tundra in central Canada. Glob Change Biol 14:740–756
Larsen KS, Jonasson S, Michelsen A (2002) Repeated freeze–thaw cycles and their effects on biological processes in two arctic ecosystem types. Appl Soil Ecol 21:187–195
Larsen KS, Grogan P, Jonasson S et al (2007) Dynamics and microbial dynamics in two subarctic ecosystems during winter and spring thaw: effects of increased snow depth. Arct Antarct Alp Res 39:268–276
Lathja K, Driscoll CT, Jarrell WM (1999) Soil phosphorus: characterization and total element analysis. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P et al (eds) Standard soil methods for long-term ecological research. Oxford University Press, Oxford
Lipson DA, Schmidt SK, Monson RK (1999) Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80:1623–1631
Lipson DA, Schmidt SK, Monson RK (2000) Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem 32:441–448
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software, Gleneden Beach, OR
McCune B, Mefford MJ (2006) PC-ORD. Multivariate analysis of ecological data. MjM Software, Gleneden Beach, OR
Michaelson GJ, Ping CL, Kling GW et al (1998) The character and bioactivity of dissolved organic matter at thaw and in the spring runoff waters of the arctic tundra north slope. Alaska J Geophys Res-Atmos 103:28939–28946
Mielke PW (1991) The application of multivariate permutation methods based on distance functions in the earth-sciences. Earth-Sci Rev 31:55–71
Miller AE, Schimel JP, Sickman JO et al (2007) Mineralization responses at near-zero temperatures in three alpine soils. Biogeochemistry 84:233–245
Morley CR, Trofymow JA, Coleman DC et al (1983) Effects of freeze–thaw stress on bacterial-populations in soil microcosms. Microb Ecol 9:329–340
Mullen RB, Schmidt SK, Jaeger CH (1998) Nitrogen uptake during snowmelt by the snow buttercup, Ranunculus adoneus. Arct Alp Res 30:121–125
Mulvaney RL (1996) Nitrogen - Inorganic Forms. In: Sparks DL (ed) Methods of soil analysis. Part 3, chemical methods. Soil Science Society of America and American Society of Agronomy, Madison, WI
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Sparks DL (ed) Methods of soil analysis. Part 3, chemical methods. Soil Science Society of America and American Society of Agronomy, Madison, WI
Nobrega S, Grogan P (2007) Deeper snow enhances winter respiration from both plant-associated and bulk soil carbon pools in birch hummock tundra. Ecosystems 10:419–431
Nobrega S, Grogan P (2008) Landscape and ecosystem-level controls on net carbon dioxide exchange along a natural moisture gradient in Canadian low arctic tundra. Ecosystems 11:377–396
Olsson PQ, Sturm M, Racine CH et al (2003) Five stages of the Alaskan Arctic cold season with ecosystem implications. Arct Antarct Alp Res 35:74–81
Petrone K, Buffam I, Laudon H (2007) Hydrologic and biotic control of nitrogen export during snowmelt: a combined conservative and reactive tracer approach. Wat Resour Res 43:W06420. doi:10.1029/2006WR005286
Polyanskaya LM, Ivanov KE, Guzev VS et al (2008) Estimation of abundance dynamics of gram-negative bacteria in soil. Microbiology 77:760–764
Schimel JP, Clein JS (1996) Microbial response to freeze–thaw cycles in tundra and taiga soils. Soil Biol Biochem 28:1061–1066
Schimel JP, Bilbrough C, Welker JA (2004) Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biol Biochem 36:217–227
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Shaver GR, Chapin FS III (1980) Response to fertilization by various plant growth forms in an Alaskan tundra: nutrient accumulation and growth. Ecology 61:662–675
Six J, Bossuyt H, Degryze S et al (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res 79:7–31
Skogland T, Lomeland S, Goksoyr J (1988) Respiratory burst after freezing and thawing of soil—experiments with soil bacteria. Soil Biol Biochem 20:851–856
Soil Classification Working Group (1998) The Canadian system of soil classification. NRC Press, Ottawa, ON
Sturm M, McFadden JP, Liston GE et al (2001) Snow–shrub interactions in Arctic tundra: a hypothesis with climatic implications. J Clim 14:336–344
Sturm M, Schimel JP, Michaelson G et al (2005) Winter biological processes could help convert arctic tundra to shrubland. Bioscience 55:17–26
Sulkava P, Huhta V (2003) Effects of hard frost and freeze–thaw cycles on decomposer communities and N mineralisation in boreal forest soil. Appl Soil Ecol 22:225–239
Tye AM, Young SD, Crout NMJ et al (2005) The fate of N-15 added to high Arctic tundra to mimic increased inputs of atmospheric nitrogen released from a melting snowpack. Glob Change Biol 11:1640–1654
Vitousek PM, Hedin LO, Matson PA (1998) Within-system element cycles, input-output budgets, and nutrient limitations. In: Pace ML, Groffman PM et al (eds) Successes, limitations and frontiers in ecosystem science. Springer-Verlag, New York
Wahren CHA, Walker MD, Bret-Harte MS (2005) Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob Change Biol 11:537–552
Walker MD, Walker DA, Welker JM et al (1999) Long-term experimental manipulation of winter snow regime and summer temperature in arctic and alpine tundra. Hydrol Process 13:2315–2330
Walker VK, Palmer GR, Voordouw G (2006) Freeze–thaw tolerance and clues to the winter survival of a soil community. Appl Environ Microb 72:1784–1792
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princetown University Press, Princetown, NJ
Weintraub MN, Schimel JP (2005) The seasonal dynamics of amino acids and other nutrients in Alaskan Arctic tundra soils. Biogeochemistry 73:359–380
Whalen SC, Cornwell JC (1985) Nitrogen, phosphorus, and organic-carbon cycling in an arctic lake. Can J Fish Aquat Sci 42:797–808
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830
Acknowledgements
We gratefully acknowledge Meghan Laidlaw, Mat Vankoughnett, Linda Cameron, Peter Lafleur, Elyn Humphreys, Mike Treberg, John Glew, Christy Barbeau, Andy Kritsch, and Bob Reid for their assistance in the field and the laboratory. We appreciate logistical support from Steve Mathews (GNWT), the Aurora Research Institute and Queen’s University, and funding support from NSERC (KB and PG), ACUNS-Garfield Weston Foundation (KB), NSTP (KB) and NSF (PG).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Buckeridge, K.M., Grogan, P. Deepened snow increases late thaw biogeochemical pulses in mesic low arctic tundra. Biogeochemistry 101, 105–121 (2010). https://doi.org/10.1007/s10533-010-9426-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9426-5