Abstract
Biodiversity differentials between macrobenthic assemblages associated with adjacent intertidal and subtidal areas of a single seagrass system were investigated for the first time. Assemblage metrics of conservation relevance—faunal abundance and its patchiness, faunal richness, and beta diversity—were examined at four contrasting dwarf-eelgrass localities in the Knysna estuarine bay, part of South Africa's Garden Route National Park but a system whose intertidal areas are heavily impacted anthropogenically. Faunal assemblages were significantly different across all localities and between subtidal and intertidal levels at each locality although their taxonomic distinctness was effectively constant. Although, as would be expected, there were clear trends for increases in overall numbers of species towards the mouth at all levels, few generalities relating to the relative importance of the subtidal seagrass habitat were evident across the whole system—magnitude and direction of differentials were contingent on locality. Shore-height related differences in assemblage metrics were minor in the estuarine and lagoonal zones but major in the marine compartment, although the much greater subtidal faunal abundance there was largely consequent on the superabundance of a single species (the microgastropod Alaba pinnae), intertidal zones then displaying the greater species diversity due to greater equitability of species densities. Along its axial channel, the Knysna subtidal seagrass does not support richer versions of the intertidal polychaete-dominated assemblages fringing it; instead, it supports different and more patchily dispersed gastropod-dominated ones. At Knysna at least, the subtidal hardly constitutes a reservoir of the seagrass biodiversity present intertidally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrass is markedly under-appreciated, for example receiving < 1.5% of the total media attention devoted to coastal systems, compared to coral reef's 72.5% (Duarte et al. 2008; Dennison 2009; van Keulen et al. 2018). Yet it plays one of the planet's most important ecosystem-service roles (Costanza et al. 2014). Although assessment of all the services provided by seagrass beds is still incomplete (Barbier et al. 2011; Nordlund et al. 2018), not least because they vary from region to region and from species to species (Nordlund et al. 2016), they have been estimated (in 2007 units) to be worth US$ 29,000 ha–1 year−1 or a total of 5–6 trillion US$ year−1—a per unit area value exceeded only by coral reefs and tidal marshes/swamps. Amongst other services (Barbier et al. 2011; Dewsbury et al. 2016), seagrasses (i) stabilise coastal sediments and prevent erosion, (ii) reduce water velocities, (iii) trap nutrients and organic molecules, (iv) shelter and feed juvenile nektonic prawns and fish of commercial importance and the adults of iconic but vulnerable dugong, green turtles and syngnathid fish; and (v) sequester carbon. Indeed, per annum, 1 ha of seagrass can sequester carbon equivalent to that emitted by a car travelling 3350 km, making it a globally significant carbon stock with an average of some 14 kg buried C m−2, and it can absorb the nutrients released in the treated effluent of 200 people (McKenzie and Yoshida 2013; Adams 2016; Lefcheck et al. 2019; Githaiga et al. 2019). In spite of these known benefits, however, anthropogenic destruction of seagrass beds continues at a very high rate, i.e. a global loss of 7% year−1 since 1990 (Waycott et al. 2009), and this could be releasing 300 Tg of blue carbon annually (Fourqurean et al. 2012).
Of all seagrass-supporting coastal systems, those under greatest threat are the shallow, sheltered, estuarine ones semi-enclosed by land (Barbier et al. 2011; Gubbay 2016). From the temperates to sub-tropics, the dominant seagrass in these is dwarf-eelgrass—species of the Zostera subgenus Zosterella in the classification accepted by the World Register of Marine Species or of Nanozostera in the recent revision of the Zosteraceae of Coyer et al. (2013). In many such areas, dwarf-eelgrass naturally carpets the intertidal zone but is replaced subtidally by other types of seagrass, for example by Zostera (subgenus Zostera) in the north temperate zone or by Cymodocea, Posidonia or Halodule in warmer regions (e.g. Lee et al. 2006; Short et al. 2007). In South Africa, however, in the absence of characteristically subtidal seagrass genera (den Hartog and Phillips 2001), the local dwarf-eelgrass species, Z. (or N.) capensis, continues into the sublittoral to depths of up to 6 m below low-water spring-tide level (LWS) (Short et al. 2011), and at Knynsa such subtidal beds occupy 130 ha or 30% of its total local area (Wasserman et al. 2020). Z. capensis is a IUCN red-listed 'vulnerable' and 'decreasing' species (Short et al. 2010, 2011) and on the basis of its acreage at Knysna (as calculated by Wasserman et al. 2020) and that for the whole of southern Africa (as estimated by Adams 2016), this one Knysna locality currently supports > 40% of its South African and > 20% of its world total, mostly in its outer marine embayment (CES 2009; Wasserman et al. 2020).
The warm-temperate Knysna estuarine bay lies within the open-access Garden Route National Park and is therefore a Protected Area under the South African National Environmental Management: Protected Areas Act, 2003 (Act No. 57 of 2003). It has not, however, been designated a Marine Protected Area, notwithstanding that it is ranked South Africa's premier estuary in terms of its biodiversity and overall conservation importance (Turpie and Clark 2007; Van Niekerk et al. 2019). Seagrasses are subject to many pressures (Gubbay 2016; Mvungi and Pillay 2019; Unsworth et al. 2019) and at Knysna two of the main ones are green-algal blooms (Human et al. 2016; Barnes 2019a; Pollard et al. 2019) and heavy exploitation for bait largely for subsistence and recreational fishing (Hodgson et al. 2000a; Napier et al. 2009; Simon et al. 2019; Claassens et al. 2020).
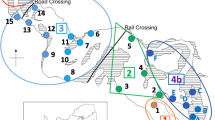
South African National Parks (SANParks) has formally declared part of the system a no-take bait reserve, but it is clear that in practice bait-harvesting takes place there too (Simon et al. 2019) (Fig. 1). Most is carried out artisanally for the mudprawn Upogebia, and Hodgson et al. (2000a) recorded some 3400 mudprawn-collection visits per annum to each of a sample of six (seagrass and non-seagrass) sites at Knysna. Their data suggest that > 30,000 plugs and corresponding holes created by 'pushing' for the prawns are scattered over the surface of each site each year (Fig. 1a–c). An even more disruptive procedure is illegal trenching (Fig. 1d) for deep-burrowing worms such as Marphysa and Gorgonorhynchus (often at night to avoid detection, especially within the no-take zone); individual trenches of up to 7 × 2 m have been recorded (Hodgson et al. 2000a).
Destructive effects of bait-collecting activities on intertidal seagrass habitat in the 'no-take bait sanctuary' section of the Knysna estuarine bay: a the hole and jettisoned plug created by pushing for mudprawns; b a substratum pock-marked by such prawn pushing and, c the resultant plugs scattered over the seagrass surface; d an area of seagrass destroyed by trenching for bait worms
Both these procedures and the associated trampling are a source of conservation concern (Mucina et al. 2006; Claassens et al. 2020), although permits are still issued for legal prawn-pumping in the Knysna seagrass outside the bait reserve. These permits restrict the number of animals that may be collected, but not the number of pumping attempts (it takes up to 10 attempts to catch each prawn; Hodgson et al. 2000a), so that the basis of the control seems to be safeguarding of the Upogebia population not of the seagrass habitat. Of the two, however, it is the Z. capensis that has by far the more limited extent and the more urgent need of conservation; the mudprawn has been described as being 'often the most abundant macrobenthic invertebrate in South African warm temperate open estuaries' (Hodgson et al. 2000b, p. 187). Unfortunately, relatively little conservation attention is directed specifically to the seagrass habitat throughout the entire Indo-West-Pacific region (Unsworth and Cullen 2010).
As in other South African estuaries (Schlacher and Wooldridge 1996), the effects on the Knysna seagrass beds of such legal and illegal bait collection extend to LWS but for practical reasons such exploitation is not conducted subtidally. Unlike some highly eutrophic estuarine bays, e.g. parts of Narragansett Bay in the USA (Thornber et al. 2017), neither do Knysna chlorophyte blooms appear to extend into the subtidal zone (Allanson et al. 2016; and pers. obs.), in some measure because although Knysna is a microtidal system (spring tidal range ≈ 1.8 m) it has a very large tidal prism (at spring tide of 19 × 106 m3) (Allanson et al. 2000), causing flushing of its main channel semi-diurnally. Therefore an important question is whether in systems like that at Knysna subtidal meadows form a natural refuge for the rich faunal biodiversity supported by seagrass.
There are immense logistic and social problems in South Africa associated with preventing illegal and restricting legal bait-harvesting, especially in areas of high unemployment where subsistence fishing provides the main or only source of protein (Branch et al. 2002; Napier et al. 2009) and the local intertidal provides the only source of bait. Therefore the natural protection of biodiversity potentially afforded by permanent water cover could make all the difference between its conservation or loss in exploitable areas. It appears, however, that comparison of the macrofaunal assemblages supported by adjacent subtidal and intertidal areas of a single seagrass species or system has never been undertaken. Hence the present study seeks for the first time to examine and quantify any differences in features of conservation relevance—faunal composition and overlap, overall abundance and its patchiness, and biodiversity—in the macrobenthic assemblages associated with adjacent inter- and subtidal sections of seagrass beds.
Methods
Sample collection and processing
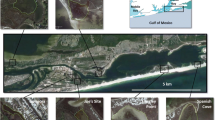
Macrofaunal sampling was conducted over a 10 week period during the 2020 austral summer in the Z. capensis beds of the Knysna estuarine bay, the research being approved by SANParks, and conducted in accordance with their scientific research regulations and requirements. Four localities on or near the main channel (Fig. 2) were sampled each at two replicate sites, c. 200 m apart, and each site comprised two immediately adjacent stations: (a) an intertidal one at or near LWS; and (b) one at a subtidal depth of some 1.5 m below LWS. Each station was represented by 16 core samples, each of 0.0054 m2 area and 100 mm depth, with samples being located some 1 m apart parallel to the shoreline. Intertidal stations were sampled before complete tidal ebb whilst the substratum was still covered by at least 15 cm of water, and the subtidal ones by snorkelling. The four localities were selected to represent areas along the long axis of the estuarine bay with contrasting hydrographic regimes (Largier et al. 2000) and significantly different intertidal seagrass macrofaunas (Barnes 2013a), i.e. Westford ('estuary'), Belvedere ('lagoon'), Brenton ('marine embayment'), and Steenbok ('sandy mouth') (see Fig. 2). All sites supported extensive swards of Z. capensis sometimes with a little Halophila ovalis, although the Westford estuarine sward was only in the form of a linear strip because of the steepness of the shore. The mixed beds of Zostera and algae (mainly Caulerpa filiformis and the alien Aspargopsis taxiformis) that dominate large areas subtidally, particularly in the lagoon (Claassens et al. 2020), were avoided because of potentially confounding variables.
Position of the study localities and of the ecological/hydrographic subdivisions along the axial channel of the Knysna estuarine bay (after Barnes 2013a, 2019b) (Google Earth Pro satellite image). The extent of seagrass cover in the mouth region varies; in 2020 very little was present seaward of the Steenbok locality
Cores were gently sieved ('puddled') through 710 µm mesh on site. This sampling procedure collects the smaller and more numerous members of the macrofauna that constitute the large majority of invertebrate biodiversity (Bouchet et al. 2002; Albano et al. 2011), though not the meiofauna nor much scarcer megafauna nor sessile animals attached to the seagrass leaves. Warwick et al. (2006) have shown that different patterning rules may apply to meiofauna and macrofauna, and likewise Davidson et al. (2004) and Leopardas et al. (2014) to sessile species. Sessile or semi-sessile species such as Halcampaster teres and Siphonaria compressa that had accidentally become detached from the seagrass leaves during sampling were therefore ignored. All intertidal stations showed clear signs of being pumped or pushed for mudprawns.
Retained material from each core: (i) was placed in a large polythene bag or bucket of local estuarine water within which all seagrass was shaken vigorously to dislodge all but sessile animals; (ii) was then re-sieved and transported immediately to a local laboratory, and (iii) was there placed in a 30 × 25 cm white tray over a light source in which the living fauna was located by visual examination using 3.5 × magnifying spectacles until no further animal could be observed.
Animals were identified to species level wherever possible, with all organismal nomenclature being as listed in the World Register of Marine Species (www.marinespecies.org), accessed March 2020, except in respect of the currently genus-less microgastropods 'Assiminea' capensis and 'A'. globulus (see Barnes 2017). It should be noted, however, that the specific identity of several animals, especially amongst the Polychaeta, is questionable because of lack of recent revision; those of South African taxa of Polycladida, Oligochaeta and Nemertini, and many members of other groups less than 3–4 mm in largest dimension, are virtually unknown. Such animals were treated as morphospecies, an operationally appropriate procedure to detect spatial patterns of numbers of species and their differential abundance (Dethier and Schoch 2006; Gerwing et al. 2020). After stage (i) above, some seagrass material from each station was retained to check whether the subtidal versus intertidal differences in dwarf-eelgrass shoot density and leaf length at Knysna conformed to the generalities documented for Z. capensis by den Hartog (1970) and later authors (e.g. Adams and Talbot 1992).
Some data on seagrass macrofaunal assemblage metrics and composition at somewhat higher shore levels, between LWS and mean low water (MLW), are also available from historical datasets at our disposal from previously published studies (Barnes and Hendy 2015a; Barnes 2019b), having recently been collected in identical fashion and at the same time of year at or near the four localities: at Westford in 2019 and at Belvedere and Brenton in 2015. Only small sparse patches of Zostera occur at higher tidal levels at Steenbok, but 2019 data are available for a nearby sandy mouth-region site at Bollard Bay, only 200 m seaward. Datasets of 32 cores per locality from these additional intertidal stations were also analysed with those specified above, i.e. forming a third tidal height at each locality.
Analyses
Numbers of each component zoobenthic species at each station and site were subjected to similarity analysis, and assemblage metrics per unit area were derived and compared via PAST 3.24 (Hammer et al. 2019), all based on abundance not occupancy data (Beck et al. 2013).
Univariate metrics assessed were: (i) overall faunal numbers per unit area, (ii) observed numbers of species per unit area, N0 [i.e. 'species density' sensu (Gotelli and Colwell 2001)], (iii) α-diversity, (iv) relative evenness (= equitability), (v) taxonomic distinctness (Δ*) (Clarke and Warwick 1998), and (vi) patchiness of overall macrofaunal abundance. Alpha-diversities were measured as Hill's N2 (Hill 1973) an 'effective number of species' measure (Daly et al. 2018) that is generally insensitive to both spatial grain/extent and size of species pool; relative evenness was measured by Pielou's J. As previously at Knysna (Barnes 2019b), patchiness in spatial abundance of the macrofaunal associations at each site and station was quantified by spatial point pattern analysis using Lloyd's (1967) index of patchiness. This index is independent of sample size over a wide range of area (Lloyd 1967) provided that the animals position themselves at random with respect to each other within a patch and that patches are large relative to sample (i.e. core) size. Statistical significance of any detected patchiness (i.e. when IP > 1) was determined by Morisita's F0 test. Correlations were assessed using Spearman's rank coefficient ρ.
Comparison of univariate assemblage metrics in adjacent intertidal and subtidal stations at the various sites used Wilcoxon matched-pairs signed rank tests with associated exact P values computed by complete enumeration of all possible reassignments of values to columns within each pair. Multivariate comparison of macrofaunal assemblage composition used hierarchical clustering analysis of S17 Bray–Curtis similarity (Legendre and Legendre 1998), ANOSIM, SIMPER, and ordination by non-metric multidimensional scaling (nMDS) carried out both on untransformed species abundances and on standardised numbers per unit area (i.e. adjusted to percentages of the unit totals) to express solely relative taxonomic composition, both with 9999 permutations. Overlaps in quantitative assemblage composition between adjacent horizons were measured by Morisita's overlap index. All multivariate analyses were based on balanced datasets with sample sizes of > 250 animals, well above the minimum number recommended by Forcino et al. (2015).
Results
Two-way ANOSIM of untransformed, of cube-root transformed, and of standardised abundances at the four localities all indicated significant dissimilarities between assemblage composition both along the estuarine axis and in relation to shore height horizon (P < 0.0001 in all cases) with locality having the greater overall effect (locality R = 0.77–0.91, position on the shore R = 0.50–0.53, dependent on transformation). The differential abundance of just five of the 116 species encountered in the seagrass (the gastropods Alaba pinnae and Nassarius kraussianus, the polychaetes Simplisetia erythraeensis and Prionospio sexoculata, and the asteroid Parvulastra exigua) were responsible for generating 54% of this overall pattern; that of A. pinnae alone accounting for 29% (SIMPER).
The greater importance of locality in influencing assemblage composition is also shown in ordination by non-metric multidimensional scaling of the data (Fig. 3). With one exception, the various sites cluster into two groups, an upstream Westford + Belvedere (estuarine + lagoonal) group and a downstream Brenton + Steenbok (marine) group; only the MLW-LWS site at Brenton is in the 'wrong' place, clustering instead with Westford and Belvedere. The same result was obtained with standardised data and after excluding from the downstream dataset the overwhelmingly local dominant Alaba. The major faunal change that occurs between Belvedere and Brenton is also highlighted by the values of one-way ANOSIM R and of Morisita's overlap index between adjacent localities shown in Table 1. The mean value of Morisita's overlap index within each of the Westford + Belvedere and the Brenton + Steenbok localities was 0.452 (SE 0.056) whereas that between the two blocks was 0.155 (SE 0.036).
Pattern of compositional similarity between the untransformed abundances of seagrass macrobenthos at the three studied shore heights at each of four localities along the Knysna estuarine bay, as indicated by ordination by non-metric multidimensional scaling of superimposed site-average S17 Bray–Curtis values. Using species abundances adjusted to percentages of each site total yielded the same pattern. Envelopes enclose sites clustered at the stated levels of Bray–Curtis similarity. Key: E—estuary (Westford), L—lagoon (Belvedere), B—bay (Brenton), M—mouth (Steenbok); 1—subtidal, 2—LWS interface, 3—intertidal
Whilst taxonomic distinctness displayed little variation with either locality or shore horizon, and species density consistently showed increasing values at all horizons from the estuarine Westford to the most marine Steenbok [the subtidal zone thus following the general axial estuarine pattern demonstrated earlier for the Knysna intertidal assemblages (Barnes 2013a)], the other assemblage metrics all also displayed a change between Belvedere and Brenton (Fig. 4). Overall abundance and macrofaunal patchiness showed relatively small and inconsistent variation down the shore in the upstream stretch, but distinctly greater values subtidally in the larger downstream section. Likewise, equitability and N2 species diversity showed relatively small and inconsistent variation upstream but at Brenton and Steenbok both species diversity and equitability were much lower in the subtidal, because of domination of the species-rich fauna by Alaba.
Relative magnitudes of the overall values per locality of the six assessed faunal assemblage metrics in the seagrass along the Knysna estuarine bay at the three shore horizons. Each column is derived from 32 core samples. Key: white bars (S)—subtidal; shaded bars (L)—LWS interface; black bars (I)—MLW-LWS intertidal
Although position along the estuarine axial gradient was clearly the dominant structuring factor, differences due to shore height were also apparent, notwithstanding that these also varied in magnitude and position from area to area (Table 1). For example, at Westford and Steenbok, the greatest change occurred between the subtidal and LWS horizons but at Belvedere and Brenton it occurred between LWS and higher up the intertidal zone. Values from the four sites showed that the subtidal horizon displayed consistently the larger levels of overall abundance, species density, taxonomic distinctness and patchiness than the adjacent LWS level (Wilcoxon W = 21–36; P < 0.04), and significantly lower values of N2 diversity and equitability (Wilcoxon W = 32–36; P < 0.03). It was notable that all observed intertidal and LWS values of patchiness were within the homogeneous range demonstrated for Knysna by Barnes (2019b), whereas those from the subtidal were well outside it, being both larger and more variable (a mean value of 1.46 and SE of 0.20). Overall, the Knysna subtidal zone supported 102 species and a faunal density of 15,900 m−2, whilst the MLW-LWS intertidal zone contained 67 species at a density of 5475 ind m−2, and the LWS interface a very similar 69 species and 5714 ind m−2. As observed elsewhere (see “Discussion” section), there were also marked and consistent differences between the nature of the Zostera leaves in the subtidal and at LWS (Wilcoxon W = 36; one-way P < 0.0004): subtidal leaves were > 35 cm long and occurred at a density < 26 core−2, whereas LWS ones were < 11 cm long and had a mean density > 47 core−2.
Faunal assemblage composition changed taxonomically with shore height. Subtidally, the microgastropod Alaba accounted for 74% of all animals; Alaba plus three other molluscs (the gastropods N. kraussianus and 'Assiminea' capensis, and the bivalve Arcuatula capensis) comprised 84% of the total; and those four together with the asteroid P. exigua formed 85.5%. Not surprisingly in the light of these numbers, molluscs contributed 86.4% of the total subtidal individuals, with crustaceans 7.3% and annelids 4.0%; and these three taxa also comprised 27%, 32% and 35% of the number of species, respectively. Although considerably less abundant upstream, the dominant Alaba occurred subtidally along the whole length of the estuarine bay. Where they co-occurred, numbers of A. pinnae correlated positively with those of the similarly microphytobenthically-grazing but larger Gibbula cicer (ρ = 0.44; P ≪ 0.00001) but negatively with those of P. exigua (ρ = − 0.43; P ≪ 0.00001) which also forages on the same type of microalgal resources. In considerable contrast, assemblages in the intertidal seagrass at MLW-LWS at the four localities were dominated by polychaetes both in terms of individual animals (46.4%) and of species (40.3%), followed by molluscs (34.6% and 25.4%, respectively), with crustaceans third with 10.6% and 25.4%. At this higher intertidal level, dominance was shared between many more species, 18 together comprising 85% of total numbers (six of the top 10 being polychaetes, of which S. erythraeensis, P. sexoculata and Caulleriella capensis were especially numerous). As might be expected, the fauna at the LWS interface was to some extent intermediate in nature, molluscs, for example, comprising 49.7% of individuals, although polychaetes showed peak numbers of species (51.9%) there; the number of species together comprising 85% of the total individuals was 17 respectively (7 polychaete, 5 mollusc and 4 crustacean).
Average SIMPER dissimilarity between the subtidal and above LWS intertidal assemblages was 88.5%, with 85% of this dissimilarity being due to the subtidal seagrass supporting more of the molluscs Alaba, Nassarius, Arcuatula and 'Assiminea', and more of the crustaceans Grandidierella, Paratylodiplax, Hymenostoma and ?Cylindroleberis, but less of the asteroid Parvulastra, of the polychaetes Simplisetia, Prionospio, Caulleriella, Paradoneis, Orbinia, Pseudopolydora and Cirriformia, of the mollusc Macoma, and of the crustacean Danielella. Of these, only Paratylodiplax was found solely in the subtidal habitat.
A particularly notable animal observed subtidally at Steenbok was the small, warm-water seagrass nerite Smaragdia souverbiana, widespread in the Indo-West Pacific but never previously recorded this far south in South Africa. Six individuals were encountered, each in a different sample, and a further two > 1 km away at another site (there together with the very poorly-known rissooid Alvania argentea), so that it was not an isolated occurrence; presumably, like other species, it has been extending its range southwards from KwaZulu Natal consequent on rising global temperatures.
Discussion
Comparison of faunal data from dwarf-eelgrass beds above and below the low tide mark is not a strictly like-with-like process, in that periodic emergence versus permanent submergence involves changes in a wide range of habitat variables besides degree of water cover. As also seen in several other seagrass genera (Apichanangkool and Prathep 2014), permanently-submerged (and/or highly sheltered) dwarf-eelgrass plants differ from periodically-emergent (and/or exposed ones) in their leaf morphology and shoot densities. Leaves of submerged and relatively sheltered ramets of Zostera (Zosterella) are considerably longer and broader (Young and Kirkman 1975; Schanz and Asmus 2003): in general, subtidal Z. capensis may have leaves > 1 m long and 2.5 mm wide, whereas those growing intertidally and in exposed conditions may be only 2 cm long and < 1 mm wide (den Hartog 1970). Characteristically, many intertidal beds also have a considerably greater shoot density (12–20 ×) than subtidal ones (Adams and Talbot 1992; Peralta et al. 2000; Lee et al. 2006; etc.). The dwarf-eelgrass plants at Knysna were typical in both these respects, and, also allowing for subtidal/intertidal differences in sediment deposition and retention (e.g. Bos et al. 2007; Braat et al. 2018) and the effects of salinity on shoot size and density and on allocation of resources to subsurface biomass (Maxwell et al. 2014), the nature of the two habitat types might be expected differentially to affect animal numbers, both of individuals and potentially of species (Stoner 1980; Attrill et al. 2000; Lee et al. 2001; Leopardas et al. 2014). Confounding variables therefore do not permit the present study to address causal hypotheses, including the specific effect of bait harvesting in structuring the Knysna macrobenthos. Nevertheless, the comparisons undertaken were sufficient to demonstrate consistent differences in faunal assemblage attributes between inter- and subtidal areas of dwarf eelgrass beds, i.e. in abundance, biodiversity and species composition, and to indicate that the magnitude of these metrics changes along the long axis of the estuarine bay. These differences were all very much more marked than those demonstrated between equivalent macrofaunal assemblages characterising different species of seagrass at a single locality in subtropical Queensland (Barnes 2020).
There was no evidence that the subtidal zone supported a qualitatively different faunal assemblage to that occurring in adjacent intertidal areas in any given region. Of the various species present in any significant numbers, only the camptandriid crab Paratylodiplax algoensis and trochid snail Gibbula cicer were found solely there, although they have occasionally been found in the low intertidal at other points and at other times in Knysna (Barnes and Ellwood 2011; Barnes and Hendy 2015a). P. algoensis is a species known largely to be restricted to subtidal regions where it appears to replace the related intertidal Danielella edwardsii (Emmerson 2016). All other species that led to the significant differences in assemblage composition showed only quantitative variation. These were not a random assortment of species, however: of the 18 most responsible for the observed difference, seven of the ten mainly intertidal animals were polychaetes and all eight of the mainly subtidal forms were molluscs or crustaceans. With one exception, these intertidally based species were also infaunal, whereas all but one of the subtidal ones were epifaunal (or possibly two, in that the precise benthic life style of the large ostracod ?Cylindroleberis is unknown). Sub-surface biomass of Z. capensis usually greatly exceeds that extending into the water column (Hanekom and Baird 1988; Paula et al. 2001), and granted that its subtidal leaves can (and at Knysna did) exceed 1 m in length (den Hartog 1970) and occurred at up to almost 5000 shoots m−2, the associated root/rhizome mass of subtidal seagrass is very large and can occupy most of the near-surface sediment. This may well account for the restriction in numbers of infaunal polychaetes subtidally (Hughes et al. 2000). Even the smaller subsurface biomass of intertidal seagrass may affect the numbers of burrowing worms in that there was a marked increase in their abundance after local loss of the eelgrass on the shores of Knysna's Steenbok Channel (Barnes 2019a; and see Pillay et al. 2010b). If that is so, then areas of subtidal seagrass are unlikely ever to provide a suitable refuge for such polychaetes.
The one exception to the epifaunal domination subtidally/infaunal domination intertidally dichotomy referred to above is the unusual epifaunal seagrass asteroid Parvulastra for which Knysna is a very important area. Although common and widespread as a rocky-shore species from the south-east Atlantic, across the Indian Ocean, to the south-west Pacific (GBIF Secretariat 2019), P. exigua is known from seagrass beds at only two sites, both in the South African Western Cape, Langebaan on the Atlantic coast (Pillay et al. 2010a) and Knysna, where it is not only present but numerous; the same disjunct distribution in South Africa as the eelgrass limpet, Siphonaria compressa (Allanson and Herbert 2005). At Knysna it is abundant only in the lagoonal reach and in some lagoon-like backwater areas of the marine embayment (see below), but there it occurs both subtidally and intertidally in densities of > 1000 m−2. Parvulastra grazes microalgal-rich biofilms (Jackson et al. 2009; Martinez et al. 2016) and this dependence on a high-light regime may account for its relative intertidal abundance.
The axial main channel provides the only subtidal habitat within the Knysna estuarine bay, but it does not support the only intertidal area of seagrass. There is an extensive block of seagrass-covered mudflats and creeks within the sheltered, saltmarsh-lined eastern half of the marine embayment, between Thesen and Leisure Islands and the mainland (see Raw et al. 2020). These seagrass beds support a qualitatively and quantitatively different faunal assemblage (Barnes 2013a). In the transitional zone between the axial channel and the system of backwater creeks, Alaba falls to a relatively insignificant 1.6% of faunal individuals, and whilst the polychaetes Simplisetia, Prionospio and Caulleriella remain dominant, as does the gastropod Nassarius, whilst the dominant amphipod is here Melita zeylanica (Barnes and Ellwood 2011; Barnes 2013b) which was not recorded from the axial channel. Further into the system of creeks and channels, Alaba drops out of the fauna altogether and, although Simplisetia, Prionospio and Melita remain, the dominant animals are now the tanaid Halmyrapseudes cooperi and the microgastropods Hydrobia knysnaensis, 'Assiminea' capensis and at higher levels 'Assiminea' globulus, these four dominants together comprising 86% of individuals (Barnes 2010; Barnes and Barnes 2014) and being largely responsible for the very high intertidal macrofaunal densities of up to more than 30,000 m−2 (Barnes 2017). The macrofaunal assemblages of relatively marine parts of the Knysna seagrass system are therefore characterised by (a) highly abundant, microgastropod-dominated macrofaunas both subtidally (i.e. by Alaba) and intertidally in the shallow backwaters (i.e. by Hydrobia and 'Assiminea'), and (b) relatively low abundance but diverse, polychaete-dominated intertidal assemblages elsewhere. There is, however, very little overlap indeed between the faunas of the sheltered, saltmarsh-enclosed eastern backwaters and those of the axial channel, and little or no representation of most intertidal backwater species in the axial subtidal. In this context, it is notable that within the Knysna seagrass system as a whole the distribution and abundance of the leaf-associated mesograzers Alaba and Parvulastra are negatively correlated, as are those of Alaba and Hydrobia, but where they do occur together, as in the backwater creeks and pools, the numbers of Hydrobia and Parvulastra may be significantly positively associated (data in Barnes 2010).
The estuarine subtidal at Knysna may be relatively protected from some of the threats affecting most of the intertidal regions, including harvesting of mudprawns, but the present results suggest that it does not appear an adequate potential refuge for the other members of Knysna's particularly rich intertidal fauna incidentally affected by such threats, except probably for Alaba in the marine embayment and possibly for Parvulastra in the lagoonal region. In many respects Knysna appears a typical, open estuary, albeit with a relatively small freshwater input, supporting a suite of macrobenthic species generally characteristic of temperate South African estuaries, lagoons and semi-enclosed bays (Schlacher and Wooldridge 1996; Teske and Wooldridge 2003; Henninger and Froneman 2011; etc.) and a seagrass fauna of a general taxonomic composition observed much more widely (Barnes and Hendy 2015b).
Neither is it alone in having a microphytobenthos-grazing population on its seagrass leaves dominated numerically by a species of the warm-temperate to tropical cerithioid microgastropod Alaba in that scattered seagrass systems as far apart as in Brazil (Cavalcante et al. 2019), Singapore (Fong et al. 2018) and tropical Queensland (Bendell 2006) show the same feature [whereas in other seagrass beds well within Alaba's climatic range it is only a minor component (Barnes 2019c)]. Knysna, however, does appear to be the only known temperate example of the phenomenon, although the related Diffalaba opiniosa can dominate the epifauna of the alga Codium on temperate rocky shores in New South Wales (Lutz et al. 2019). Like A. pinnae, other Alaba species also seem to be mainly shallow-subtidal in nature, often extending upshore into the low intertidal as well (Bendell 2006; Prozorova et al. 2010). Unfortunately, the Knysna A. pinnae is a poorly known species that seems endemic to South Africa. It is described as being a 'common' estuarine gastropod along southern and eastern shores in the monograph of Kilburn and Rippey (1982), yet strangely it appears to be missing from published faunal surveys of other South African estuaries with the exception of the nearby Swartvlei (Whitfield 1988, 1989) and KwaZulu's estuarine Lake St Lucia in which Perissinotto et al. (2014) record it as occurring sporadically. It is not mentioned at all in works treating the nature of the South African estuarine fauna, e.g. by Day (1981), De Villiers and Hodgson (1999) or Perissinotto et al. (2013). This may be because of confusion with Assiminea, a genus widely used as a catch-all for any South African estuarine microgastropod (Barnes 2017). In spite of the uncertainty associated with A. pinnae, however, there seems little reason to suppose that the relationship between subtidal and intertidal seagrass assemblages at Knysna is in any manner aberrant, and what is the case there probably applies equally to other bays and estuaries. Subtidal seagrass supports its own biodiverse and abundant assemblage of macrofaunal species, with those that particularly characterise the intertidal zone occurring at low density and seeming more likely to be 'stragglers' or incidental members of the fauna below LWS. It is certainly not an extension of the seagrass system occurring intertidally. The only caveat here is that what applies to Zostera-dominated systems may not be the case in stands of other seagrass genera, in that it has recently been shown in subtropical Queensland that whereas, all other things being equal, the macrofaunal assemblages associated with sympatric intertidal Cymodocea serratula, Halodule uninervis and Halophila ovalis were very similar, all three differed from that supported by adjacent areas of intertidal Zostera (Zosterella) muelleri (Barnes 2020).
Supplementary data
The data sets generated by this and earlier studies have been lodged in electronic format in the Rondevlei Office of SANParks Scientific Services (https://dataknp.sanparks.org/sanparks/metacat/Nerinak.23.11/sanparks) and are available on request.
References
Adams JB (2016) Distribution and status of Zostera capensis in South African estuaries—a review. S Afr J Bot 107:63–73
Adams JB, Talbot MMB (1992) The influence of river impoundment on the estuarine seagrass Zostera capensis Setchell. Bot Mar 35:69–75
Albano PG, Sabelli B, Bouchet P (2011) The challenge of small and rare species in marine biodiversity surveys: microgastropod diversity in a complex tropical coastal environment. Biodivers Conserv 20:3223–3237
Allanson BR, Herbert DG (2005) A newly discovered population of the critically endangered false limpet Siphonaria compressa Allanson, 1958 (Pulmonata: Siphonariidae), with observations on its reproductive biology. S Afr J Sci 101:95–97
Allanson BR, Maree B, Grange N (2000) An introduction to the chemistry of the water column of the Knysna Estuary with particular reference to nutrients and suspended solids. Trans R Soc S Afr 55:141–162
Allanson BR, Human LRD, Classens L (2016) Observations on the distribution and abundance of a green tide along an intertidal shore, Knysna Estuary. S Afr J Bot 107:49–54
Apichanangkool P, Prathep A (2014) Changes in seagrass leaf reddening and morphology in response to emersion. Bot Mar 57:443–440
Attrill MJ, Strong JA, Rowden AA (2000) Are macroinvertebrate communities influenced by seagrass complexity? Ecography 23:114–121
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
Barnes RSK (2010) Spatial variation in abundance and diversity of the smaller surface and near-surface eelgrass-associated intertidal macrobenthos within a warm-temperate estuarine bay in the Garden Route National Park, RSA. Aquat Conserv 20:762–772
Barnes RSK (2013a) Distribution patterns of macrobenthic biodiversity in the intertidal seagrass beds of an estuarine system, and their conservation significance. Biodivers Conserv 22:357–372
Barnes RSK (2013b) Spatial stability of macrobenthic seagrass biodiversity. Mar Ecol Progr Ser 493:127–139
Barnes RSK (2017) Little-known and phylogenetically obscure South African estuarine microgastropods (Mollusca: Truncatelloidea) as living animals. J Nat Hist 57:87–113
Barnes RSK (2019a) Context dependency in the effect of Ulva-induced loss of seagrass cover on estuarine macrobenthic abundance and biodiversity. Aquat Conserv 29:163–174
Barnes RSK (2019b) Local patchiness of macrobenthic faunal abundance displays homogeneity across the disparate seagrass systems of an estuarine bay. Mar Environ Res 148:99–107
Barnes RSK (2019c) Spatial structure of a multi-species guild: the dominant biofilm-grazing microgastropods of seagrass. Hydrobiol 827:293–307
Barnes RSK (2020) Do different sympatric seagrasses support macrobenthic faunas of differing composition, abundance, biodiversity or patchiness? Mar Environ Res 160:104983
Barnes RSK, Barnes MKS (2014) Biodiversity differentials between the numerically-dominant macrobenthos of seagrass and adjacent unvegetated sand in the absence of sandflat bioturbation. Mar Environ Res 99:34–43
Barnes RSK, Ellwood MDF (2011) The significance of shore height in intertidal macrobenthic seagrass ecology and conservation. Aquat Conserv 21:614–624
Barnes RSK, Hendy IW (2015a) Seagrass-associated macrobenthic functional diversity and functional structure along an estuarine gradient. Estuar Coast Shelf Sci 164:233–243
Barnes RSK, Hendy IW (2015b) Functional uniformity underlies the common spatial structure of macrofaunal assemblages in intertidal seagrass beds. Biol J Linn Soc 115:114–126
Beck J, Holloway JD, Schwanghart W (2013) Undersampling and the measurement of beta diversity. Methods Ecol Evol 4:370–382
Bendell BE (2006) Interactions amongst invertebrates, epiphytes, and seagrasses in tropical intertidal meadows. PhD thesis, James Cook University. https://eprints.jcu.edu.au/15488. Accessed Mar 2020
Bos AR, Bouma TJ, de Kort GLJ, van Katwijk MM (2007) Ecosystem engineering by annual intertidal seagrass beds: sediment accretion and modification. Estuar Coast Shelf Sci 74:344–348
Bouchet P, Lozouet P, Maestrati P, Heros V (2002) Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol J Linn Soc 75:421–436
Braat L, Leuven JRFW, Lokhorst IR, Kleinhans MG (2018) Effects of estuarine mudflat formation on tidal prism and large-scale morphology in estuaries. Earth Surf Proc Land 44:417–432
Branch GM, May J, Roberts B, Russell E, Clark BM (2002) Case studies on the socio-economic characteristics and lifestyles of subsistence and informal fishers in South Africa. S Afr J Mar Sci 24:439–462
Cavalcante LL, Barroso CX, de Carneiro PBM, Matthews-Cascon H (2019) Spatiotemporal dynamics of the molluscan community associated with seagrass on the western equatorial Atlantic. J Mar Biol Ass UK 99:1285–1294
CES (2009) Knysna toll highway EIA volume 3. Final Knysna environmemntal impact report. Coastal and Environmental Services, Grahamstown
Claassens L, Barnes RSK, Wasserman J, Lamberth SJ, Miranda NAF, van Niekerk L, Adams JB (2020) Knysna estuary health: ecological status, threats and options for the future. Afr J Aquat Sci 45:65–82
Clarke KR, Warwick RM (1998) A taxonomic distinctiveness index and its statistical properties. J Appl Ecol 35:523–531
Costanza R, de Groot R, Sutton P, Van der Ploeg D, Anderson SJ, Kubiszewski I, Farber S, Turner RK (2014) Changes in the global value of ecosystem services. Global Environ Change 26:152–158
Coyer JA, Hoarau G, Kuo J, Tronholm A, Veldsink J, Olsen JL (2013) Phylogeny and temporal divergence of the seagrass family Zosteraceae using one nuclear and three chloroplast loci. Syst Biodivers 11:271–284
Daly AJ, Baetens JM, De Baets B (2018) Ecological diversity: measuring the unmeasurable. Mathematics. https://doi.org/10.3390/math6070119
Davidson IC, Crook AC, Barnes DKA (2004) Quantifying spatial patterns of intertidal biodiversity: is movement important? Mar Ecol 25:15–34
Day JH (1981) The estuarine fauna. In: Day JH (ed) Estuarine ecology with particular reference to southern Africa. Balkema, Rotterdam, pp 147–178
De Villiers CJ, Hodgson AN (1999) Studies on estuarine macroinvertebrates. The macrobenthos. In: Allanson BR, Baird D (eds) Estuaries of South Africa. Cambridge University Press, Cambridge, pp 167–191
den Hartog C (1970) The sea-grasses of the world. North Holland, Amsterdam
den Hartog C, Phillips RC (2001) Common structures and properties of seagrass beds fringing the coasts of the world. In: Reise K (ed) Ecological comparisons of sedimentary shores. Springer, Heidelburg, pp 195–212
Dennison WC (2009) Global trajectories of seagrasses, the biological sentinels of coastal ecosystems. In: Duarte CM (ed) Global loss of coastal habitats: rates, causes and consequences. Fundacion BBVA, Madrid, pp 91–107
Dethier MN, Schoch GC (2006) Taxonomic sufficiency in distinguishing natural spatial patterns on an estuarine shoreline. Mar Ecol Progr Ser 306:41–49
Dewsbury BM, Bhat M, Fourqurean JW (2016) A review of seagrass economic valuations: gaps and progress in valuation approaches. Ecosyst Serv 18:68–77
Duarte CM, Dennison WC, Orth RJW, Carruthers TJB (2008) The charisma of coastal ecosystems: addressing the imbalance. Estuar Coast 31:233–238
Emmerson WD (2016) A Guide to, and Checklist for, the Decapoda of Namibia, South Africa and Mozambique, Volume 3. Cambridge Scholars, Newcastle upon Tyne
Fong JM, Lai S, Yaakub SM, Ow YX, Todd PA (2018) The diet and feeding rates of gastropod grazers in Singapore's seagrass meadows. Bot Mar 61:181–192
Forcino FL, Leighton LR, Twerdy P, Cahill JF (2015) Reexamining sample size requirements for multivariate, abundance-based research: when resources are limited, the research does not have to be. PLoS ONE 10(6):e0128379. https://doi.org/10.1371/journal.pone.0128379
Fourqurean JW et al (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509
GBIF Secretariat (2019) Parvulastra exigua (Lamarck, 1816). Global Biodiversity Information Facility Backbone Technology. Checklist dataset https://doi.org/10.15468/39omei. Accessed Mar 2020
Gerwing TG, Cox K, Allen Gerwing AM, Campbell L, Macdonald T, Dudas SE, Juanes F (2020) Varying intertidal invertebrate taxonomic resolution does not influence ecological findings. Estuar Coast Shelf Sci 232:106516
Githaiga MN, Frouws AM, Kairo JG, Huxham M (2019) Seagrass removal leads to rapid changes in fauna and loss of carbon. Front Ecol Evol 7:62
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gubbay S et al (2016) European red list of habitats. Part 1. Marine habitats. European Union, Luxembourg
Hammer Ø, Harper DAT, Ryan PD (2019) PAST: Paleontological statistics software package for education and data analysis, Version 3.24
Hanekom N, Baird D (1988) Distribution and variations in seasonal biomass of eelgrass Zostera capensis in the Kromme estuary, St Francis Bay, South Africa. S Afr J Mar Sci 7:51–59
Henninger TO, Froneman PW (2011) Macrofaunal community structure in the littoral zone of a freshwater-deprived, permanently open Eastern Cape estuary. Afr Zool 46:263–279
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hodgson AN, Allanson BR, Cretchley R (2000a) The exploitation of Upogebia africana (Crustacea: Thalassinidae) for bait in the Knysna Estuary. Trans R Soc S Afr 55:197–204
Hodgson AN, Allanson BR, Cretchley R (2000b) An estimation of the standing stock and population structure of Upogebia africana (Crustacea: Thalassinidae) in the Knysna Estuary. Trans R Soc S Afr 55:187–196
Hughes RG, Lloyd D, Ball L, Emson D (2000) The effects of the polychaete Nereis diversicolor on the distribution and transplanting success of Zostera noltii. Helgol Mar Res 54:129–136
Human LRD, Adams JB, Allanson BR (2016) Insights into the cause of an Ulva lactuca bloom in the Knysna Estuary. S Afr J Bot 107:55–62
Jackson AC, Murphy R, Underwood AJ (2009) Patiriella exigua: grazing by a starfish in an overgrazed intertidal system. Mar Ecol Progr Ser 376:153–163
Kilburn R, Rippey E (1982) Sea shells of southern Africa. Macmillan SA, Johannesburg
Largier JL, Attwood C, Harcourt-Baldwin J-L (2000) The hydrographic character of the Knysna Estuary. Trans R Soc S Afr 55:107–122
Lee SY, Fong CW, Wu RSS (2001) The effect of seagrass (Zostera japonica) canopy structure on associated fauna: a study using artificial seagrass units and sampling of natural beds. J Exp Mar Biol Ecol 259:23–30
Lee SY, Kim JB, Lee SM (2006) Temporal dynamics of subtidal Zostera marina and intertidal Zostera japonica on the southern coast of Korea. Mar Ecol 27:133–144
Lefcheck JS, Hughes BB, Johnson AJ, Pfirrman BW, Rasher DB, Smyth AR, Williams BL, Beck MW, Orth RJ (2019) Are coastal habitats important nurseries? A meta-analysis. Conserv Lett 2019:e12645. https://doi.org/10.1111/conl.12645
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
Leopardas V, Uy W, Nakaoka M (2014) Benthic macrofaunal assemblages in multispecific seagrass meadows of the southern Philippines: variation among vegetation dominated by different seagrass species. J Exp Mar Biol Ecol 457:71–80
Lloyd M (1967) Mean crowding. J Anim Ecol 36:1–30
Lutz ML, Minchinton TE, Davis AR (2019) Differences in architecture between native and non-indigenous macroalgae influence associations with epifauna. J Exp Mar Biol Ecol 514–515:76–86
Martinez AS, Byrne M, Coleman RA (2016) What and when to eat? Investigating the feeding habits of an intertidal herbivorous starfish. Mar Biol 163:166
Maxwell PS, Pitt KA, Burfeind DD, Olds AD, Babcock RC, Connolly RM (2014) Phenotypic plasticity promotes persistence following severe events: physiological and morphological responses of seagrass to flooding. J Ecol 102:54–64
McKenzie LJ, Yoshida RL (2013) Seagrass Watch: proceedings of workshop for monitoring seagrass habitats in South East Queensland, August 2013. Seagrass Watch HQ, Cairns.
Mucina L, Adams JB et al (2006) Coastal vegetation of South Africa. Strelitzia 19:660–696
Mvungi EF, Pillay D (2019) Eutrophication overrides warming as a stressor for a temperate African seagrass (Zostera capensis). PLoS ONE 14(4):e0215129. https://doi.org/10.1371/journal.pome.0215129
Napier VR, Turpie JK, Clark BM (2009) Value and management of the subsistence fishery at Knysna Estuary, South Africa. Afr J Mar Sci 31:297–310
Nordlund LM, Koch EW, Barbier EB, Creed JC (2016) Seagrass ecosystem services and their variability across genera and geographical regions. PLoS ONE 11(10):e0163091. https://doi.org/10.1371/journal.pone.0163091
Nordlund LM, Jackson EL, Nakaoka M, Samper-Villarreal J, Beca-Carretero P, Creed JC (2018) Seagrass ecosystem services—what's next? Mar Poll Bull 134:145–151
Paula J, Fidalgo e Costa P, Martins A, Gove D (2001) Patterns of abundance of seagrasses and associated infaunal communities at Inhaca Island, Mozambique. Estuar Coast Shelf Sci 53:307–318
Peralta G, Pérez-Lloréns JL, Hernández I, Brun F, Vergara JJ, Bartual A, Gálvez GCM (2000) Morphological and physiological differences between two morphotypes of Zostera noltii Hornem. from the south-western Iberian peninsula. Helgol Mar Res 54:80–86
Perissinotto R, Stretch DD, Taylor RH (2013) Ecology and conservation of Estuarine systems. Lake St Lucia as a global model. Cambridge University Press, New York
Perissinotto R, Miranda NAF, Raw JL, Peer N (2014) Biodiversity census of Lake St Lucia, iSimangaliso wetland park (South Africa): gastropod molluscs. ZooKeys 440:1–43
Pillay D, Branch GM, Steyn A (2010a) Unexpected effects of starfish grazing on sandflat communities following an outbreak. Mar Ecol Progr Ser 398:173–182
Pillay D, Branch GM, Griffiths CL, Williams C, Prinsloo A (2010b) Ecosystem change in a South African marine reserve (1960–2009): role of seagrass loss and anthropogenic disturbance. Mar Ecol Progr Ser 415:35–48
Pollard M, Whitfield AK, Hodgson AN (2019) Possible influences of a macroalgal bloom in eelgrass beds on fish assemblages in the lower Knysna Estuary, South Africa. Afr J Aquat Sci 43:319–323
Prozorova LA, Sitnikova TY, Noseworthy R, Kashin IA, Zvyagintsev AY (2010) On the morphology and taxonomy of Pacific gastropods in families of tropical origin Litiopidae and Dialidae (Caenogastropoda: Cerithioidea). In: Dautova TN, Lutaenko KA (eds) Proceedings of the international conference on marine biodiversity of east Asian seas: status, challenges and sustainable development. Premium Press, Vladivoskok, pp 138–141
Raw JL, Riddin T, Wasserman J, Lehman TWK, Bornman TG, Adams JB (2020) Salt marsh elevation and responses to future sea-level rise in the Knysna Estuary, South Africa. Afr J Aquat Sci 45:49–64
Schanz A, Asmus H (2003) Impact of hydrodynamics on development and morphology of intertidal seagrasses in the Wadden Sea. Mar Ecol Progr Ser 261:123–134
Schlacher TA, Wooldridge TH (1996) Axial zonation patterns of subtidal macrozoobenthos in the Gamtoos Estuary, South Africa. Estuaries 19:680–696
Short F, Carruthers T, Dennison W, Waycott M (2007) Global seagrass distribution and diversity: a bioregional model. J Exp Mar Biol Ecol 350:3–20
Short FT et al (2010) Zostera capensis. The IUCN Red List of Threatened Species 2010:e.T173370A7001305. https://doi.org/10.2305/IUCN.UK.2010-3.RLTS.T173370A7001305.en
Short FT et al (2011) Extinction risk assessment of the world's seagrass species. Biol Conserv 144:1961–1971
Simon C, du Toit AN, Smith MKS, Claassens L, Smith F, Smith P (2019) Bait collecting by subsistence and recreational fishers in Knysna Estuary may impact management and conservation. Afr Zool 54:91–103
Stoner AW (1980) The role of seagrass biomass in the organization of benthic macrofaunal assemblages. Bull Mar Sci 30:537–551
Teske PR, Wooldridge TH (2003) What limits the distribution of subtidal macrobenthos in permanently open and temporarily open/closed South African estuaries? Salinity versus. sediment particle size. Estuar Coast Shelf Sci 57:225–238
Thornber CS, Guidone M, Deacutis C, Green L, Ramsay CN, Palmisciano M (2017) Spatial and temporal variability in macroalgal blooms in a eutrophied coastal estuary. Harmful Algae 68:82–96
Turpie J, Clark B (2007) Development of a conservation plan for temperate South African estuaries on the basis of biodiversity importance, ecosystem health and economic costs and benefits. Final Report. Cape Town: Anchor Environmental Consultants/C.A.P.E. Regional Estuarine Management Programme
Unsworth RKF, Cullen LC (2010) Recognising the necessity for Indo-Pacific seagrass conservation. Conserv Lett 3:63–73
Unsworth RKF, McKenzie LJ, Collier CJ, Cullen-Unsworth LC, Duarte CM, Eklöf JS, Jarvis JV, Jones BJ, Nordlund LM (2019) Global challenges for seagrass conservation. Ambio 48:801–815
Van Keulen M, Nordlund LM, Cullen-Unsworth LC (2018) Towards recognition of seagrasses and their sustainable management. Mar Poll Bull 134:1–4
Van Niekerk L, Adams JB, Lamberth SJ, MacKay CF, Taljaard S, Turpie JK, Weerts SP, Raimondo DC (eds) (2019) South African National Biodiversity Assessment 2018: Technical Report. Volume 3: Estuarine Realm. South African National Biodiversity Institute, Pretoria, Report SANBI/NAT/NBA2018/Vol3/A
Warwick RM, Dashfield SL, Somerfield PJ (2006) The integral structure of a benthic infaunal assemblage. J Exp Mar Biol Ecol 330:12–18
Wasserman J, Claassens L, Adams JB (2020) Mapping subtidal estuarine habitats with a remotely operated underwater vehicle (ROV). Afr J Mar Sci 42:123–128
Waycott M et al (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA 106:12377–12381
Whitfield AK (1988) The fish community of the Swartvlei Estuary and the influence of food availability on resource utilization. Estuaries 11:160–170
Whitfield AK (1989) The benthic invertebrate community of a southern Cape estuary: structure and possible food sources. Trans R Soc S Afr 47:159–179
Young PC, Kirkman H (1975) The seagrass communities of Moreton Bay, Queensland. Aquat Bot 1:191–202
Acknowledgements
We are most grateful to: Rhodes University Research Committee for financial support; the Rondevlei Office of SANParks Scientific Services and the Knysna Area Manager, Megan Taplin, for permission to undertake fieldwork in the Knysna Section of the Garden Route National Park under Permit BARN-RSK/2019-034; Dai Herbert for suggesting the identity of Alvania argentea; and Ruth Farre and Riyad de Klerk, South African Navy Hydrographic Office, for advance information on the Knysna Tide Tables.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Angus Jackson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Coastal and marine biodiversity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barnes, R.S.K., Claassens, L. Do beds of subtidal estuarine seagrass constitute a refuge for macrobenthic biodiversity threatened intertidally?. Biodivers Conserv 29, 3227–3244 (2020). https://doi.org/10.1007/s10531-020-02019-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-02019-0