Abstract
Alien predators can have large impacts on prey. It is important that we understand, and ideally predict, these impacts. Here, we compare predatory impacts of size-matched decapod crustaceans—invasive alien Eriocheir sinensis and Pacifastacus leniusculus, and native European Austropotamobius pallipes—and use this case study to inform methods for impact prediction. We quantify functional responses (FRs) on three macroinvertebrate prey species, examine switching behaviour, and measure metabolic rates as a possible mechanistic explanation for differences in predation. FRs show a consistent pattern: attack coefficients and maximum feeding rates are ordered E. sinensis ≥ P. leniusculus ≥ A. pallipes for all prey species. Attack coefficients of E. sinensis are up to 6.7 times greater than those of size-matched crayfish and maximum feeding rates up to 3.0 times greater. FR parameters also differ between the invasive and native crayfish, but only up to 2.6 times. We find no evidence of switching behaviour in crayfish but suggestions of negative switching in E. sinensis. Differences in FR parameters are mirrored by differences in routine, but not standard, metabolic rate. Overall, our data predict strong predatory impacts of E. sinensis, even relative to alien P. leniusculus. Strong impacts of P. leniusculus relative to A. pallipes may be driven more by body size or abundance than per capita effect. FRs vary between prey types in line with existing knowledge of impacts, supporting the use of FRs in quantitative, prey-specific impact predictions. MRs could offer a general mechanistic explanation for differences in predatory behaviour and impacts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a fundamental ecological interaction that can influence population dynamics and community structure (Wellborn et al. 1996; Chesson 2000; Hatcher et al. 2014). As well as informing basic ecological understanding, quantifying predatory interactions can inform applied management decisions, for example in the context of biocontrol (Symondson et al. 2002), conservation biology (Sutherland 1998) and biological invasions. The success and impact of alien species are often driven by resource use (Catford et al. 2009; Dick et al. 2014), and in particular by predatory interactions (Davis 2003; Salo et al. 2007; Sax and Gaines 2008). Thus, a quantitative understanding of predation by alien species is an important step in understanding and predicting impact in biological invasions (Dick et al. 2014) and consequently allocating limited management resources appropriately (Kumschick et al. 2012).
Predation can be described by a functional response (FR): the relationship between prey density N0 and the rate of prey consumption Ne (Holling 1959). Holling’s disk equation (Eq. 1) provides a simple functional response model.
There are two key FR parameters. Handling time (h) is the time needed for a predator to kill, ingest and digest a prey item. The attack coefficient (a) is the rate of successful search by a predator, or a measure of the rate of attacks that end in capture (Jeschke et al. 2002). Handling time is inversely related to the height of an FR curve, which describes the maximum possible rate at which prey can be consumed by a predator. Short handling times and correspondingly high FR curves are suggestive of high per capita impacts, which may translate into large population impacts in the field (Dick et al. 2013, 2017; Dodd et al. 2014; Xu et al. 2016). The attack coefficient defines the shape of an FR curve. When the attack coefficient is constant across prey densities, the FR is an asymptotically-declining Type II curve. Type II FRs are likely to be associated with the most severe impacts on prey populations because predation pressure remains high even at low prey densities (Murdoch and Oaten 1975; Juliano 2001). In contrast, a sigmoid Type III curve is generated when the attack coefficient is, at low prey densities, positively associated with prey density. Such a positive association could be mediated by predator learning, changes in foraging tactics, changing stimuli from prey, or structural complexity of habitats (Murdoch and Oaten 1975; Alexander et al. 2012).
When more than one prey type is present, attack coefficients can change with prey density if predators switch between prey types, attacking a more abundant prey type disproportionately more often that would be expected based on its abundance (Murdoch 1969). Detecting Type III FRs, or the factors that might lead to them, is important because they fundamentally change the consequences of predation for populations. For example, switching can reduce the likelihood of prey population extinction: because rare prey are attacked disproportionately infrequently, a low density refuge from predation is created (Murdoch 1969). However, switching could also maintain long-term predation pressure by sustaining predator populations when any single prey type becomes rare.
Metabolic rates (MRs) could provide a mechanistic explanation for predatory impacts. Metabolism refers to the enzymic processing of energy and materials within living organisms. MRs determine the rate of all biological activities (Brown et al. 2004). Generally, MR and food consumption should be positively associated: species with a high MR will require more food to fuel that metabolism, but may also be able to catch and process food more rapidly (Careau et al. 2008; Biro and Stamps 2010). In terms of FR parameters, a high MR necessitates a high maximum feeding rate (Rall et al. 2012) but could facilitate a shorter handling time (e.g. more energy available to fuel digestion) and a higher attack coefficient (e.g. more available energy to fuel movement and therefore encounter rates; Dell et al. 2014). Two fundamental measures of MR in ectotherms are standard (SMR) and routine (RMR). SMR reflects energy processing under minimal functional activity i.e. the minimum necessary to sustain life, or the idling cost of the individual’s metabolic engine (largely the viscera). RMR reflects energy processing incorporating SMR and spontaneous, voluntary movements (Cech and Brauner 2011). To get an accurate picture of any feeding-metabolism relationship, it is important to measure both SMR and RMR: theoretically, one or the other or both could be related to feeding rate, depending on the predator’s behaviour and physiology (Careau and Garland 2012).
Crustaceans are particularly successful as alien species and can exert strong impacts through a variety of mechanisms, including predation (Strayer 2010; Hänfling et al. 2011). In particular, decapod crustaceans are some of the most widely distributed and high-impact aliens in fresh waters (Karatayev et al. 2009; Strayer 2010). As flexible omnivores, they can impart impacts through predatory behaviour. Globally, two of the most successful and damaging alien decapods are the American signal crayfish Pacifastacus leniusculus (Dana 1852) and the Chinese mitten crab Eriocheir sinensis Milne Edwards 1853. Both species are biologically invasive having spread across large areas outside their native range, both can reach high densities in their novel range, and both have substantial ecological or economic impacts (Laverty et al. 2015).
Pacifastacus leniusculus is native to parts of North America but has been introduced and become a pest across much of Europe (Souty-Grosset et al. 2006). Eriocheir sinensis is native to the north-western Pacific but has been transported around the world, with key established populations on the west coast of the USA and in north-west Europe (Dittel and Epifanio 2009). Invasion by P. leniusculus can change community structure through a combination of competition, disease transmission and resource consumption (Crawford et al. 2006; Dunn et al. 2008; Twardochleb et al. 2013; Mathers et al. 2016). Evidence from mesocosms and field manipulations suggests E. sinensis may cause similar declines in macroinvertebrate populations through predation (Yu and Jiang 2005; Rudnick and Resh 2005; Rosewarne et al. 2016). However, our knowledge of these predatory impacts and their underlying mechanisms remains incomplete, especially for E. sinensis (Rosewarne et al. 2016).
Across Europe, the native white-clawed crayfish Austropotamobius pallipes (Lereboullet 1858) has declined over the past 40 years as alien crayfish, including P. leniusculus, have expanded their range (Dunn et al. 2008; Holdich et al. 2009; Füreder et al. 2010). More recent advancement of E. sinensis populations has created zones of overlap with P. leniusculus, and sympatry between E. sinensis and A. pallipes is also possible (Rosewarne et al. 2016). Thus, it is important to understand the relative ecological impacts of these species to appreciate how ecosystems have changed (or might change) as these species meet and replace each other. As a low-impact native analogue, A. pallipes also provides a baseline to contextualise the impact of the alien species.
Here, we aim to quantify the relative predatory impacts of A. pallipes, P. leniusculus and E. sinensis and investigate a possible mechanistic explanation for any differences. First, we compare laboratory-derived FRs on three macroinvertebrate prey types of differing morphology and behaviour (an amphipod crustacean, chironomid larvae and a gastropod mollusc). Predatory impacts may vary among prey species, so assessing FRs across a variety of prey species is important (Dick et al. 2014; Dodd et al. 2014). Second, we examine predation when more than one prey type is present—specifically the tendency of the predators to switch between similarly sized gastropods and amphipods. Third, we compare MRs (derived from oxygen consumption rates) between the three decapod species. We hypothesise that the alien species will have higher FR curves than A. pallipes in line with other invasive alien-native comparisons, and will show a greater tendency to switch between prey since diet flexibility may be a common trait of successful alien species (Sol et al. 2002; Weis 2010). We expect interspecific differences in MRs to mirror differences in feeding rates.
Methods
Experimental animals and husbandry
Decapods were collected by hand from established populations in the UK between 2013 and 2016. A. pallipes were collected from Adel Beck, Leeds (lat 53°51′18′′N, long 1°34′′26′′W) under licenses from Natural England (#20131266 and #20144477). P. leniusculus were collected from Fenay Beck, Huddersfield (lat 53°38′29′′N, long 1°43′51′′W) under agreement with the UK Environment Agency. Eriocheir sinensis were collected from the River Thames at Chiswick (lat 51°29′17′′N, long 0°14′44′′W) under agreement with the Port of London Authority. The three experiments (FR, switching and MR) were run at different times on different batches of decapods, but all three species were tested simultaneously within each experiment.
Stock decapods were kept in a controlled environment room in the University of Leeds, at 14 ± 0.2 °C (range) and under a 12:12 h light:dark cycle, for at least 2 weeks before use to allow acclimation to laboratory conditions and reduce the influence of any wild environmental cues (e.g. tidal cycles for E. sinensis; Gilbey et al. 2008). Stock tanks were communal by species, contained aerated aged tap water with excess PVC piping as shelter, and were supplied with Hikari® Crab Cuisine™ pellets and dried leaf litter (abscised Acer pseudoplatanus L. leaves) ad libitum.
A week before use in experiments, decapods were measured and isolated in individual plastic tanks (23 cm length, 15 cm width, 8 cm depth, with translucent white lids and sides covered in black plastic to minimise visual disturbance). Each tank was constantly aerated and contained one black PVC shelter (10 cm length, 5 cm diameter). Isolated animals were fed a standardised diet: four Hikari® Crab Cuisine™ pellets every other day, followed by starvation for 24 h before feeding experiments and 48 h before MR measurements.
Within each experiment, decapods were matched by overall body size (Section S1, Supplementary Information). We defined decapod body size as the first component from a principal components analysis on body mass and cmax (maximum carapace dimension: carapace length to tip of rostrum for crayfish; carapace width for crabs), explaining 88.7% of the variance in these parameters. Consequently, crabs were slightly heavier but shorter (cmax) than crayfish of similar body size. Across all experiments, mean ± SE decapod masses were: A. pallipes 10.6 ± 0.4 g; P. leniusculus 10.5 ± 0.3 g; E. sinensis 12.6 ± 0.4 g. Mean ± SE cmax was: A. pallipes 32.3 ± 0.4 mm; P. leniusculus 32.8 ± 0.3 mm; E. sinensis 30.9 ± 0.3 mm (see Table S1 for measurements of decapods used in each experiment).
Decapods were matched by overall body size for the feeding experiments because both the mass and body dimensions of predators relative to prey can affect predatory impact (Holling 1964; Nilsson and Brönmark 2000; Rall et al. 2012). Because the aim of this study was to relate MRs and predatory behaviour, we then analysed MRs of animals with a similar body size and mass to those used in FR experiments (Eq. 7). For other purposes, it may be more appropriate to compare mass-specific feeding and metabolic data (i.e. scaled to a common body mass). These analyses are presented in the Supplementary Information (Sections S5, S7 and S8). For the present study, they yield similar conclusions to analyses based on size-specific data.
Decapods used in experiments were in good condition (all limbs intact, no injuries to body) and free of visible parasites (Souty-Grosset et al. 2006). No decapods moulted within a week of use in any experiment, and typically not within two weeks. A mixture of male and female decapods of each species was used. Non-reproductive behaviours are generally similar between sexes in sub-adult crabs and crayfish (Taylor 2016).
For feeding experiments, three different prey species were used, chosen to represent differing motility and physical defence. Amphipods Dikerogammarus villosus (Sowinsky 1894) were collected from Grafham Water, Cambridgeshire (lat 52°17′52′′N, long 0°18′44′′W). Gastropods Bithynia tentaculata (L. 1758) were sourced from laboratory stocks, originating from various water bodies around Leeds. Chironomid larvae were sourced from a pet retailer in Leeds. For each prey species, animals in good condition and of similar size (Table 1) were blindly and haphazardly allocated to decapod predators. Uneaten and uninjured prey were returned to communal tanks and re-used.
Functional responses
Experimental design
FR data were obtained by providing an individual decapod with a known density of prey, allowing it to feed for 24 h and then calculating consumption based on the amount of prey remaining. FR experiments were run in the same controlled environment room as the stock tanks i.e. 14 ± 0.2 °C (range) and 12:12 h light:dark cycle.
Individual experimental tanks (dimensions as for isolation tanks) were set up containing three litres of aged tap water, approximately 150 glass stones (20 mm diameter, 9 mm height) to provide some structural complexity (Alexander et al. 2012) and a designated number of prey animals (Table 1). After 1 h to allow prey to settle, a single decapod was transferred from its isolation tank to each experimental tank.
After a 24 h feeding period, each tank was destructively sampled and remaining prey counted. We distinguished live prey, dead but complete prey, and identifiable parts of prey (fractions of animals). Consumption was calculated as the number of prey supplied minus all remaining flesh (whole and damaged prey). Killing was defined as prey that had been wholly or partially consumed i.e. excluding dead but undamaged prey assumed to reflect background mortality. Controls, to check background mortality, were tanks with prey but no predator (three replicate tanks per prey type per density, excluding chironomids at a density of 1200 tank−1).
Predators were re-used at different prey densities until each predator species x prey density combination was replicated five (B. tentaculata prey) or six times (chironomid and D. villosus prey). Re-use led to pseudoreplication, but was a constraint enforced by the use of Endangered A. pallipes. However, no individual animal was used more than once at any prey density, and no more than eight times in total. Experimental design also minimised the influence of re-use on results. First, initial predator hunger levels were standardised by the set feeding/starvation schedule. Between uses, predators were returned to isolation tanks, fed with the standard ration (four Crab Cuisine™ pellets) for 24 h then starved for 24 h. Second, across uses of individual predators, the order of presentation of prey densities was randomised. Third, replicates were roughly blocked by time, such that within each block one replicate was run for all predator species x prey density combinations (except for 1200 chironomids.tank−1, an additional density tested after all others).
For logistical reasons and because of seasonal prey availability, each prey item was tested over a 1–2 month period at different times of year (D. villosus Nov–Dec, chironomids Jan–Feb; B. tentaculata Jun–Jul).
Statistical methods
All statistical analyses were carried out in R version 3.3.1 (R Core Team 2016) with α = 0.05 unless otherwise specified. We present analyses conducted using number of prey consumed as the response variable: because it is only prey consumed that fuel metabolic demand, this metric is more relevant than the number of prey killed when comparing predator physiology and there is partial consumption of prey (Section S4). Additional analyses using prey killed as the response variable, which is more relevant when considering effects on prey populations, yielded similar overall results (Section S4).
For each predator x prey species combination, FR type was determined by logistic regression (with quasibinomial errors) following Juliano (2001) and Alexander et al. (2012). Where results were ambiguous, fits for different FR types were compared using Akaike’s Information Criterion (AIC) (Pritchard et al. 2017). Based on these analyses, all FRs were modelled as Type II curves. Maximum likelihood model fitting and parameter estimation were performed within the R package frair (frair::frair_fit; Pritchard et al. 2017) and used Rogers’ random predator equation (Eq. 2; Rogers 1972) which modifies Holling’s disk equation (Eq. 1) to account for the non-replacement of prey within trials.
where Ne is the number of prey consumed or killed, N0 is the initial density of prey (prey.tank−1), a is the attack coefficient (tanks.day−1), h is the handling time (days.prey item−1) and T is the total time available for predation (days). In practice, the Lambert W function is incorporated into Eq. 2 to make it solvable (Bolker 2008).
To visualise variability around fitted FR curves, 95% BCa confidence intervals were drawn from bootstrap populations generated from the original data (frair::frair_boot; n = 1999). Following Juliano (2001), parameters were compared using indicator variables (frair::frair_compare). Because multiple pairwise comparisons were made within each prey type, significance was considered against Holm-Bonferroni corrected α values (Holm 1979).
Switching
Experimental design
The potential for predators to switch between alternative prey items depending on their relative density was investigated by presenting predators with D. villosus and B. tentaculata at a range of relative abundances. These prey items were chosen because they are similar in mass (Table 1) and will not prey upon each other.
Switching experiments followed a similar protocol to FR experiments (isolation and feeding, settlement of prey items in tanks with glass beads, similarly sized prey, same temperature and light regime, destructive sampling after 24 h feeding). The most important difference was that two prey types were presented simultaneously. A total of 280 individual prey were added to tanks at one of the following five ratios (D. villosus to B. tentaculata): 0.15:0.85, 0.35:0.65, 0.50:0.50, 0.65:0.35 or 0.85:0.15. As a further difference to the FR experiments, three days before experimental feeding each decapod was allowed to feed on 10 D. villosus, then two days before use allowed to feed on 10 B. tentaculata. Only individuals that consumed each prey type were used in switching experiments, such that all individuals had recent experience feeding on both prey types.
In the switching experiment, individual predators were only used once to ensure feeding was not differentially influenced by prior experience. Five replicates were run at each ratio for A. pallipes, six for P. leniusculus and eight for E. sinensis. Five controls, with no decapod predator, were run at the equal ratio (140 D. villosus and 140 B. tentaculata) to check prey survival.
Statistical methods
As for FRs, we present analyses using prey consumed (rounded to the nearest whole individual) as the response variable. Additional analyses carried out using prey killed as the response variable yielded similar results (Section S6).
Mean prey consumption (total number of individuals of both prey types) was compared between decapod species, using a quasipoisson generalised linear model and post hoc Tukey contrasts with Holm-Bonferroni adjustment of p values (multcomp::glht; Hothorn et al. 2016).
To detect switching, the observed proportions of prey in predator diets were compared with null proportions (assuming the absence of switching). This analysis used population proportions i.e. consumption by all predators of a species at each relative density. First, for each decapod species, electivity towards D. villosus, c, was determined using Eq. 3 (Murdoch and Oaten 1975).
where NDv and NBt are the total number of D. villosus and B. tentaculata consumed, by all predators of a species, when prey were equally available (ratio 0.50:0.50). A. pallipes did not consume any B. tentaculata in this situation, so an arbitary value of NBt = 1 was used to allow estimation of c. A value of c = 1 indicates no electivity (prey are consumed in equal numbers); c > 1 indicates electivity towards D. villosus and c < 1 electivity towards B. tentaculata. We describe c as electivity rather than preference, as it does not necessarily depend on a behavioural choice by the predator (Murdoch 1969).
Second, for each decapod species and at each relative prey density, the expected proportion of D. villosus in the predator diet under the null hypothesis of no switching, PDv (null), was calculated using Eq. 4 (Murdoch and Oaten 1975).
where FDv is the proportion of D. villosus in the available food. Expected numbers of D. villosus and B. tentaculata in predator diets were then calculated, using PDv (null) and observed total consumption. Finally, expected and observed prey numbers were compared using Fisher’s exact tests (fisher.test). If the proportion of D. villosus in the diet was lower than the null proportion when D. villosus was relatively rare, but higher than the null when D. villosus was relatively common, we would conclude that switching had occurred (Murdoch 1969).
The above calculations assume that absolute and relative prey densities do not change over time: a reasonable assumption for our data. The high prey densities ensured that in 83% of trials < 20% of the prey were consumed (and in 99% of trials < 30% of prey were consumed) and wide spacing of relative prey densities meant that final relative densities never became more extreme than adjacent starting densities. Neither prey species was completely consumed in any replicate trial. We also note that these tests will be subject to high Type I error rates: there is variation around c (because it is estimated from sample data) that is not incorporated into estimates of null consumption. However, given limited significance in the results this does not affect our overall conclusions.
Metabolic rates
Experimental design
As a proxy for MRs, oxygen consumption rates (ṀO2) of individual decapods were measured in a custom made intermittent-flow respirometer (following Quetin 1983 and Svendsen et al. 2016; see Section S2 for diagram). In brief, the respirometer was a PVC food storage container that was airtight when clipped shut and enclosed 505 ml of water. The chamber contained a magnetic stir bar to mix water during measurements and a PVC shelter (6 cm length, 4.5 cm diameter) to minimise stress. An optical dissolved oxygen (DO) probe (YSI ProODO, YSI Incorporated, OH) was inserted into the chamber through a rubber seal. Plastic mesh separated the decapod from the stir bar and DO sensor cap. One piece of inflow silicone tubing (40 mm length, 3 mm internal diameter) connected the chamber to a flush pump (Sacem BIP 4W) via an air trap, whilst another 40 mm length of tubing provided an outflow. The chamber and attachments were submerged in a water bath, which was constantly aerated and contained a combined filter/ultraviolet light (All Pond Solutions, Middlesex, UK) to continually mix the water bath and minimise microbial growth. The entire setup was housed in an incubator with the same temperature (14.0 ± 0.3 °C range) and photoperiod (12:12 h) as the controlled environment room. Housing in a separate incubator ensured complete standardisation of visual and acoustic cues during measurements.
Prior to measurement, decapods were isolated for one week and fed a standardised diet (as for FR experiments), including a 48 h starvation period before measurement to minimise the influence of digestive processes on MR. An individual animal was transferred in water (to avoid introducing air bubbles) to the respirometer at 20:00 h. After a 5 h acclimation period, which allowed ṀO2 to stabilise, measurements were taken every 20 min (E. sinensis) or 30 min (crayfish) within automated 50 min cycles (Section S2). Temperature- and pressure-compensated [DO] (mg O2 L−1), along with temperature (°C) and pressure (mmHg) separately, were logged every 20 s via YSI’s Data Manager Software. At the same time, animals were recorded by webcam (Logitech Pro 9000 and Webcam XP 5 software). Eighteen cycles were completed for each animal: nine in the light and nine in the dark. E. sinensis were allocated a shorter measurement phase than crayfish because pilot studies suggested their ṀO2 was higher. The chosen measurement phase durations ensured oxygen pressures in the respirometer never dropped below 80% but R2 values of fitted lines (see below) remained high (≥ 0.88) even when ṀO2 was low.
Due to equipment limitations, only one individual could be measured per day. MR was measured for eight A. pallipes, 12 P. leniusculus and 10 E. sinensis. The order in which individuals of each species were tested was randomised to remove any confounding temporal effects. To minimise microbial growth, respirometry equipment was scrubbed in a weak (0.5%) bleach solution and allowed to dry between uses.
Statistical methods
For each individual, [DO] measurements over time were split by eye into the longest possible linear sections. A least-squares regression line (with R2 ≥ 0.88) was fitted to each section in Microsoft Excel. Some short sections (≤ 3 min) with unstable [DO] readings, and thus poor regression fits, were omitted from analyses. ṀO2 for each section was calculated according to Eq. 5, suitable for closed-system respirometers (adapted from Cech and Brauner 2011):
where ṀO2 is oxygen consumption rate (mg O2 h−1), m is the gradient of the linear decline in oxygen concentration (mg O2 L−1 s−1), Vt is the total volume of the respirometer chamber (0.505 L) and Vc is the volume of each individual crayfish (determined by displacement immediately after ṀO2 measurement). ṀO2 was uncorrected for background respiration, as controls (respirometer with no decapod) indicated this was negligible.
Each individual’s lowest recorded ṀO2 across all sections was taken as an estimate of its SMR. Where possible, webcam recordings were used to verify that this coincided with a period of minimal activity. Two E. sinensis were probably active during all measurements, so SMR was not recorded. Each individual’s RMR was estimated as a weighted average of ṀO2 values across all sections, overall and separately for the light and dark phases (Eq. 6):
where ṀO2(s) is the oxygen consumption rate for section s, ts is the duration of section s, and T is the total duration of all sections. Thus, RMR incorporates periods of activity as well as periods of rest.
MR and ṀO2 are strongly mass-dependent (Cech and Brauner 2011). In order to interpret consumption data from FR experiments, MRs were adjusted to the mean mass of animals used in FR trials using Eq. 7 (adapted from Cech and Brauner 2011).
where massFR is the mean mass (g) of each species across all FR trials (A. pallipes 10.4 g, P. leniusculus 10.1 g, E. sinensis 12.0 g), massMR is the mass (g) of an individual animal used in metabolism experiments, and b is a scaling exponent for MR against mass. In the absence of a complete set of species- and rate-specific values for b, all adjustments were made using b= 0.71 based on the field MR of Orconectes rusticus crayfish (McFeeters et al. 2011).
Mass-adjusted MRs (Eq. 7) were compared between species using ANOVA and post hoc Tukey contrasts with Holm-Bonferroni correction of p values (multcomp::glht). We initially built full models containing species and sex and their interaction, but these were simplified by stepwise deletion of terms to contain species only (Crawley 2007). Within species, mass-adjusted diurnal and nocturnal MRs were compared using paired t tests.
Results
Functional responses
Prey survivorship in the presence of decapods was significantly lower than survivorship in control treatments (D. villosus 75.9% vs. 97.1%, chironomids 37.5% vs. 94.5%, B. tentaculata 83.6% vs. 97.4%; χ2 tests for these overall proportions and for each decapod species separately all p< 0.001). Thus, we infer that the decapods were acting as predators (not just scavenging dead prey) in the experimental arenas. Predation was also directly observed in separate tanks.
Using prey consumption as the response variable, FRs for all predator x prey species combinations were best described by a Type II curve. In most logistic regressions of proportional consumption against prey density, the first order term was significantly negative (Table S3.1). In two regressions, where the first order term was negative but not significantly different from zero (E. sinensis consuming chironomid larvae p = 0.169 and A. pallipes consuming B. tentaculata p = 0.050), AIC values were lower for Type II than Type I fits.
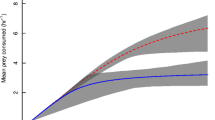
Across all prey items, E. sinensis had a significantly greater attack coefficient than both crayfish species (z tests, p≤0.012 for all comparisons): at least 2.2 times that of A. pallipes on all prey types, and between 1.2 (on chironomids) and 4.1 (on B. tentaculata) times that of P. leniusculus (Table 2). In addition, the attack coefficient of P. leniusculus was at least 1.7 times greater than that of A. pallipes on all prey items, and always significantly greater (z tests, p≤ 0.007 for all comparisons). Higher attack coefficients are manifested as steeper initial rises in FR curves (i.e. greater predation rates at low prey densities; Fig. 1).
Functional response curves of size-matched A. pallipes (green areas, solid lines), P. leniusculus (blue areas, dashed lines) and E. sinensis (orange areas, dotted lines) on (a) D. villosus; n = 6 per density (b) chironomid larvae; n = 6 per density (c) B. tentaculata; n = 5 per density. Curves were modelled in frair using Rogers’ random predator equation. Shaded areas show 95% bootstrapped BCa confidence intervals for each curve
Eriocheir sinensis had a high maximum feeding rate (1/hT) on all prey items, by virtue of its short handling time (Table 2). The maximum feeding rate of E. sinensis was significantly higher than the maximum feeding rate of both crayfish species when D. villosus or chironomid larvae were prey (z tests, p< 0.001 for all comparisons; Table S3.2): at least 2.9 times higher on D. villosus (72 vs. 24 − 25 amphipods.day−1) and at least 1.9 times higher on chironomid larvae (647 vs. 303 − 346 chironomids.day−1). With B. tentaculata as prey, E. sinensis had a higher feeding rate than A. pallipes, but not significantly so (22 vs. 18 snails.day−1, z = 1.49, p = 0.136) and a similar feeding rate to P. leniusculus (22 snails.day−1, z = − 0.02, p = 0.984). Considering the two crayfish species, P. leniusculus had a higher maximum feeding rate than A. pallipes on all prey items (1.03 times higher on D. villosus, 1.1 times higher on chironomid larvae and 1.3 times higher on B. tentaculata; Table 2), but only significantly so on chironomid larvae (z = 6.39, p < 0.001; Table S3.2).
Switching
Prey survivorship in controls, containing 140 of each prey animal, was high (D. villosus 96.8% and B. tentaculata 99.7%). Thus, as for FR experiments, we infer that the decapods were acting as predators (not just scavenging dead prey) in the experimental arenas.
In the switching experiments, E. sinensis consumed significantly more prey in total (across all relative densities mean ± SE individuals consumed = 50.3 ± 3.2) than P. leniusculus (18.1 ± 1.7) and A. pallipes (18.6 ± 1.1) (Tukey adjusted p < 0.001 for both). The crayfish species did not differ in the total number of prey consumed (Tukey adjusted p = 0.883).
All decapods showed strong electivity towards D. villosus when both prey types were equally common: D. villosus formed a significantly greater proportion of the diet than would be expected under random feeding (A. pallipes c = 96.0; P. leniusculus c = 26.3; E. sinensis c = 16.1; binomial tests of proportion of D. villosus in diet = 0.5, p < 0.001 for all three predator species). As electivity ≠ 1, the null hypothesis for switching (Eq. 4) yields a non-linear curve on a plot of proportional consumption against availability of D. villosus (Fig. 2). The observed proportion of D. villosus in the diet did not differ from null expectations for either crayfish species at any prey density (Fig. 2). For E. sinensis, the observed proportion of D. villosus in the diet only differed from null expectations at one relative prey density (0.35; Fisher’s exact test p = 0.016).
Proportion of D. villosus in the diet of size-matched decapod predators at varying relative densities of D. villosus to B. tentaculata. At all relative densities, total prey density was fixed at 280 tank−1. Note that the y axes begin at 0.6. Points are population proportions with 95% binomial confidence intervals. Curves are expected proportions in the absence of preference, based on consumption when prey types are equally available. Asterisk indicates significant deviation from null hypothesis (Fisher’s exact tests on numbers of prey consumed, without correction for multiple testing)
Metabolic rates
Here we present analyses using MRs adjusted to the mass of animals used in FR experiments (Eq. 7). These are therefore MRs of decapods with a similar body size, but not a similar mass. For comparisons of MRs scaled to a common mass, see Section S8.
Mean ± SE SMRs were A. pallipes 0.31 ± 0.04 mg O2 h−1, P. leniusculus 0.30 ± 0.02 mg O2 h−1, E. sinensis 0.34 ± 0.03 mg O2 h−1. There was no difference in these SMRs between the three decapod species (Fig. 3a; ANOVA F2,25 = 0.68, p = 0.515).
Mass-adjusted (to mean mass of animals used in FR experiments) oxygen consumption rates of decapod crustaceans, as proxies for metabolic rates. a Standard metabolic rate (SMR): the lowest recorded ṀO2 associated with minimal activity b diurnal routine metabolic rate (RMR): a weighted average of all ṀO2 measurements during the light phase and c nocturnal RMR: a weighted average of all ṀO2 measurements during the dark phase. Letters indicate significant differences (within panels) based on Tukey contrasts with Holm-Bonferroni correction of p values. Bars show means ± 2 SE. P. len.—Pacifastacus leniusculus
In contrast to SMR, RMR (calculated across both day and night) did differ between species (ANOVA F2,27 = 15.61, p< 0.001). Mean ± SE RMRs were A. pallipes 0.50 ± 0.06 mg O2 h−1, P. leniusculus 0.75 ± 0.08 mg O2 h−1, E. sinensis 1.25 ± 0.13 mg O2 h−1. The RMR of E. sinensis was signifcantly greater than that of P. leniusculus (1.7 times higher; Tukey adjusted p = 0.004), which in turn had a significantly greater RMR than A. pallipes (1.5 times higher; Tukey adjusted p = 0.015).
RMRs of the alien species were significantly higher at night than during the day (E. sinensis paired t = 3.09, df = 9, p= 0.013; P. leniusculus t = 4.83, df = 11, p<0.001), whilst the RMR of A. pallipes was marginally lower at night than during the day (t = − 2.02, df = 7, p = 0.083). Consequently, during the day RMR did not differ between the crayfish (Fig. 3b; Tukey adjusted p = 0.61) but E. sinensis had a higher RMR than both crayfish species (Tukey adjusted ps ≤ 0.002; overall ANOVA F2,27 = 11.74, p < 0.001). At night, RMR differed between all species pairs (Fig. 3c; Tukey adjusted ps ≤ 0.037; overall ANOVA F2,27 = 21.53, p< 0.001).
Discussion
This paper combines experimentally determined FRs, switching behaviour and MRs to understand the predatory impacts of freshwater decapod crustaceans. We provide quantitative data on the relative impact of important invasive alien species and a native non-invasive analogue. Our data highlight the potential for strong, previously underappreciated predatory impacts by E. sinensis. Our data suggest differences in activity levels (reflected in RMR) could provide a mechanistic explanation for differences in predatory consumption and impacts of alien species.
Our FR experiments, supported by total consumption in our switching experiments, indicate that E. sinensis is a more voracious predator than both native and alien crayfish. Rosewarne et al. (2016) reported that E. sinensis had a higher FR than P. leniusculus and A. pallipes. However, we demonstrate that relative impact of E. sinensis may be much greater than previously thought, with an attack coefficient up to 6.7 times, and maximum feeding rate up to 3.0 times, that of a similarly sized crayfish (Table 2). Our data also suggest the relatively high impact of E. sinensis is conserved across prey types. This is clearly true for amphipods and chironomids. There was a similar trend for gastropods, although maximum feeding rate on these thick-shelled, operculate snails was limited somewhat by the time taken to extract and ingest the flesh (pers. obs.; Mills et al. 2016). In the field, strong predation pressure from E. sinensis whether prey are abundant (small h) or rare (large a) could lead to prey population decline or extinction. Interestingly, when prey species are themselves alien, predation by E. sinensis could provide biotic resistance to subsequent invasions (Twardochleb et al. 2012).
Considering the crayfish species, our FR data suggest per capita predation by alien P. leniusculus consistently exceeds that of A. pallipes on a range of prey types. Pacifastacus leniusculus had a significantly higher attack coefficient than A. pallipes on all prey items, reflecting a steeper initial rise of the FR curve—even with the constraints on the curves at low densities imposed by our non-replacement design (Dick et al. 2014). Thus, our data suggest P. leniusculus is a more effective predator when prey are rare, and will exert high predation pressure when prey populations are most vulnerable to additional mortality (Murdoch and Oaten 1975). Alien P. leniusculus also had a higher maximum feeding rate than A. pallipes on all prey items, in accord with previous studies using G. pulex as prey (Haddaway et al. 2012; Rosewarne et al. 2016) and the general pattern emerging from FR studies in invasion ecology (Dick et al. 2017). However, this difference was only significant on chironomid prey, and differences were generally small in magnitude (up to 1.3 times higher in P. leniusculus) relative to the differences observed between E. sinensis and the two crayfish species.
Differences in FRs on each prey species also match previous observations of predatory impact. For example, E. sinensis had an especially high maximum feeding rate on amphipods: at least 2.9 times greater than the crayfish. Accordingly, in mesocosm experiments amphipods were the only prey group that E. sinensis affected more strongly than P. leniusculus (Rosewarne et al. 2016). Amphipods and other motile taxa may be amongst the least affected by crayfish in the field (Crawford et al. 2006; Mathers et al. 2016), so we would expect their FRs to be low. Eriocheir sinensis also had a relatively high feeding rate on chironomid larvae: at least 1.9 times greater than the crayfish. In field or mesocosm studies, chironomids have been found to be strongly affected by E. sinensis (Yu and Jiang 2005; Rudnick and Resh 2005; Czerniejewski et al. 2010) but are amongst the macroinvertebrate taxa least affected by crayfish predation (Nyström et al. 1996; Twardochleb et al. 2013; but see Crawford et al. 2006). Meanwhile, the decapod species had more similar maximum feeding rates on gastropod prey (Table 2). This agrees with field or mesocosm observations that gastropods are amongst the macroinvertebrates least affected by E. sinensis predation (Yu and Jiang 2005) and most affected by crayfish predation (Lodge et al. 1994; Twardochleb et al. 2013), and that the decapods may have similar overall impacts on gastropod populations (Rosewarne et al. 2016). The FR of E. sinensis on gastropods may be low compared to its FR on amphipods or chironomids whilst the FR of the crayfish may be relatively high, bringing the crab and crayfish FRs closer together for gastropods than for other prey types.
Through its effects on both predator and prey behaviour, structural habitat complexity can modify the shape of FRs. In particular, it often reduces predation rates at low prey densities—by disrupting predator movement, providing a physical refuge for prey or facilitating camouflage—to generate a Type III FR (Alexander et al. 2012; Barrios-O’Neill et al. 2015). However, there was no evidence of this effect in our experiments. FRs were Type II, as in previous experiments of decapod predation in simple habitats (Haddaway et al. 2012; Rosewarne et al. 2016). The structural complexity we provided may have had no effect on predator or prey behaviour (e.g. the decapods were large enough to walk over the beads, and could reach through gaps with their legs or pereopods) or may have even facilitated predation at low prey densities (e.g. by restricting prey movement).
Our data support the use of FRs as a simple, cost-effective tool for rapid assessment of invader impacts, as explained by Dick et al. (2014) and supported by the analysis of Dick et al. (2017) in which high impact alien species had higher FRs than native analogues in 18 of 22 studied consumer-resource pairs. At one level, our data support the use of comparative FRs on a single prey type to rapidly score impact potential, because similar conclusions regarding relative FR shape and height were drawn for all of our prey types. At another level, because the details of our FRs were sensitive to prey type in accord with observations in more natural situations, our data support the use of FRs to make specific predictions about magnitude of impact on different prey groups (Dick et al. 2013; Dodd et al. 2014). However, further field data would be useful to verify this relationship.
Although simple FRs (based on individual, size-matched predators feeding on single prey types) are a robust starting point for predicting alien species’ impacts, several additional factors could modulate the field impacts of our focal decapods—generally or in specific contexts. First, interspecific differences in both body size and abundance could augment the per capita effect of E. sinensis and P. leniusculus relative to A. pallipes (Parker et al. 1999; Pintor et al. 2009). The alien decapods grow to larger sizes than A. pallipes (Souty-Grosset et al. 2006; Dittel and Epifanio 2009). Larger animals generally eat more, owing to positive relationships between body size and traits such as metabolic rate, reaction distance and exploratory speed (Brown et al. 2004; Rall et al. 2012; Hirt et al. 2017). Second, aquatic alien species reach higher densities than natives on average (Hansen et al. 2013), and this is probably the case for E. sinensis and P. leniusculus relative to A. pallipes (Guan 2000; Demers et al. 2003; Rudnick et al. 2003). The impact of a population of predators generally increases with abundance (Parker et al. 1999), although the effect may be less than additive if mutual interference reduces the per capita impact of individual predators (Pintor et al. 2009; Médoc et al. 2013). Third, predatory impacts might be affected by the consumption of non-animal food sources (Médoc et al. 2018). All of our studied decapods are opportunistic omnivores, consuming leaf litter and other detritus even when animal prey are present (Bondar et al. 2005; Rudnick and Resh 2005; Haddaway et al. 2012; Rosewarne et al. 2016), although the precise balance between predation, herbivory and detritivory may be context-dependent (Larson et al. 2017). Future work should quantify how predatory FRs are affected by these factors—and others such as temperature, structural complexity, higher predators and parasites—that were beyond the scope of the present study.
Data on predator switching could complement single-species FRs to improve impact predictions. Switching—changing electivity towards prey types as their relative densities change—was not definitively observed in any of our decapod predator species. The proportion of D. villosus and B. tentaculata in crayfish diets always matched null expectations, assuming no switching. This implies that in the field, predation pressure on a single prey type could be maintained even when it becomes rare, potentially leading to local prey extinction (Murdoch and Oaten 1975): switching will not temper impacts on any single prey species. Eriocheir sinensis may exert particularly strong predation pressure on rare prey since it showed a tendency towards negative prey switching i.e. higher than expected proportional consumption of D. villosus when it is the less abundant prey (see also Section S7: smaller crabs with longer gastropod handling times showed the tendency even more clearly). Negative switching could help to explain the large impacts of E. sinensis on mesocosm populations of amphipods (Rosewarne et al. 2016). However, we encourage further investigation of switching with prey items that are more similar in defence strategies and handling time, thus eliciting weaker predator electivity. Switching may be more likely in such situations (Murdoch and Oaten 1975).
Our data indicate that that MRs could provide a mechanistic explanation for the observed differences in feeding rates and, by extension, differences in impacts of alien species on prey populations. There were large interspecific differences in the RMR of similarly sized decapods: E. sinensis had a greater RMR than the crayfish species, and P. leniusculus had a greater RMR than A. pallipes (driven by greater nocturnal activity). These results also held when MRs were adjusted to a common mass (Section S8). Together, our RMR and FR data indicate positive associations between the supporting traits of activity, RMR and feeding rate across species. An active species with a high RMR both needs to feed at a higher rate and is able to feed at a higher rate: it needs to fuel the high rate of energy processing, but is able to do so because it has more energy available for movement (which could increase encounter rates and attack coefficients; Dell et al. 2014; Hirt et al. 2017) and more energy available for physiological processes such as digestion (which could reduce handling times; Millidine et al. 2009). Accordingly, observed interspecific differences in RMR match the rank order of differences in feeding rate (cf. Careau et al. 2008; Rall et al. 2012), whilst webcam recordings suggest that the differences in RMR were related to activity in the respirometer. The higher RMR of E. sinensis and P. leniusculus at night is also consistent with their known nocturnal activity (Styrishave et al. 2007; Gilbey et al. 2008), and may be associated with higher predatory impacts on nocturnal than diurnal prey. We acknowledge confinement in a respirometer may have influenced activity levels and hence RMR (Careau et al. 2008; Toscano and Monaco 2015), so encourage further investigation of activity in more natural scenarios.
In contrast to RMR, SMR did not significantly differ between size-matched decapods (again, this was also true for mass-matched decapods; Section S8). Furthermore, differences in SMR were small in magnitude (E. sinensis SMR only around 1.1 times that of the crayfish) relative to differences in maximum feeding rate (at least 1.9 times on amphipods and chironomids). Thus SMR and RMR are apparently unrelated across the decapod species, suggesting the core metabolic engine (providing the energy for vital bodily functions and tissue maintenance) runs at a similar rate in all the species and supporting our inference that high feeding rates were associated with activity and metabolism above and beyond SMR. In other words, the maximum rate of energy processing is independent of the size of core metabolic engine (independent model of Careau and Garland 2012). Note the implication for explaining species’ impacts or interactions using MR: rates that include activity, such as field or RMRs, should be more closely related to real-world impacts than basal or SMRs (e.g. Lohr et al. 2017).
As well as being related to impact, FRs and MRs might be related to invasion success (Lagos et al. 2017), although less strongly and in variable directions. High resource consumption rates, sometimes measured explicitly as FRs, are associated with success of alien species at various stages of the invasion process (Catford et al. 2009; Xu et al. 2016; McKnight et al. 2017). High MRs might be linked to fast life history traits that can confer invasion success e.g. high activity levels, faster growth and greater reproductive rates (Lindqvist and Huner 1999; Sakai et al. 2001; Ricklefs and Wikelski 2002; McKnight et al. 2017). However, species with a fast life history, linked to high MRs and/or FRs, could struggle to invade marginal environments where resources are not abundant. Invasions might be transient if a species’ high energetic requirement reduces its ability to tolerate stressful periods (Alpert 2006). Perhaps the high FR and MR of E. sinensis contributes to its observed boom and bust population dynamics (Rudnick et al. 2003)?
Quantitative evidence of alien species’ impacts is an important factor for making decisions about their management (Kumschick et al. 2012). Our data provide novel evidence for two important invasive alien decapods in Europe. Eriocheir sinensis and P. leniusculus had consistently high predatory impacts on a range of macroinvertebrate prey relative to the impact of A. pallipes, associated with differences in RMR. The difference in per capita effect between the crayfish species was relatively small, although P. leniusculus could have a stronger impact in the field owing to its greater abundance and/or body size. Meanwhile, the per capita effect of E. sinensis was exceptionally high on soft-bodied prey and it showed some evidence of negative switching onto soft-bodied prey, highlighting predation as an underappreciated mechanism by which E. sinensis could cause large impacts. Data from more natural settings are desirable, but our laboratory data support the use of FRs, and potentially RMRs, as part of a toolbox to predict and understand alien species’ impacts.
References
Alexander M, Dick J, O’Connor N et al (2012) Functional responses of the intertidal amphipod Echinogammarus marinus: effects of prey supply, model selection and habitat complexity. Mar Ecol Prog Ser 468:191–202
Alpert P (2006) The advantages and disadvantages of being introduced. Biol Invasions 8:1523–1534
Barrios-O’Neill D, Dick JTA, Emmerson MC et al (2015) Predator-free space, functional responses and biological invasions. Funct Ecol 29:377–384
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol Evol 25:653–659
Bolker BM (2008) Ecological models and data in R. Princeton University Press, Princeton
Bondar CA, Bottriell K, Zeron K, Richardson JS (2005) Does trophic position of the omnivorous signal crayfish (Pacifastacus leniusculus) in a stream food web vary with life history stage or density? Can J Fish Aquat Sci 62:2632–2639
Brown JH, Gillooly JF, Allen AP et al (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Careau V, Garland T Jr (2012) Performance, personality and energetics: correlation, causation and mechanism. Physiol Biochem Zool 85:543–571
Careau V, Thomas D, Humphries MM, Réale D (2008) Energy metabolism and animal personality. Oikos 117:641–653
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40
Cech JJJ, Brauner CJ (2011) Techniques in whole animal respiratory physiology. In: Farell AP (ed) Encyclopedia of fish physiology: from genome to environment. Elsevier, London, pp 846–853
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Evol Syst 31:343–366
Crawford L, Yeomans WE, Adams CE (2006) The impact of introduced signal crayfish Pacifastacus leniusculus on stream invertebrate communities. Aquat Conserv Mar Freshw Ecosyst 16:611–621
Crawley MJ (2007) The R book. Wiley, Chichester
Czerniejewski P, Rybczyk A, Wawrzyniak W (2010) Diet of the Chinese mitten crab, Eriocheir sinensis H. Milne Edwards, 1853, and potential effects of the crab on the aquatic community in the River Odra/Oder estuary (N.-W. Poland). Crustaceana 83:195–205
Davis MA (2003) Biotic globalization: does competition from introduced species threaten biodiversity? Bioscience 53:481–489
Dell AI, Pawar S, Savage VM (2014) Temperature dependence of trophic interactions are driven by asymmetry of species responses and foraging strategy. J Anim Ecol 83:70–84
Demers A, Reynolds JD, Cioni A (2003) Habitat preference of different size classes of Austropotamobius pallipes in an Irish River. Bull Français la Pêche la Piscic 370:127–137
Dick JTA, Gallagher K, Avlijas S et al (2013) Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol Invasions 15:837–846
Dick JTA, Alexander ME, Jeschke JM et al (2014) Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol Invasions 16:735–753
Dick JTA, Laverty C, Lennon JJ et al (2017) Invader relative impact potential: a new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. J Appl Ecol 54:1259–1267
Dittel AI, Epifanio CE (2009) Invasion biology of the Chinese mitten crab Eriocheir sinensis: a brief review. J Exp Mar Bio Ecol 374:79–92
Dodd JA, Dick JTA, Alexander ME et al (2014) Predicting the ecological impacts of a new freshwater invader: functional responses and prey selectivity of the 'killer shrimp', Dikerogammarus villosus, compared to the native Gammarus pulex. Freshw Biol 59:337–352
Dunn JC, McClymont HE, Christmas M, Dunn AM (2008) Competition and parasitism in the native white clawed crayfish Austropotamobius pallipes and the invasive signal crayfish Pacifastacus leniusculus in the UK. Biol Invasions 11:315–324
Füreder L, Gherardi F, Holdich D et al (2010) Austropotamobius pallipes. The IUCN red list of threatened species 2010:e.T2430A9438817. Accessed 18 Jul 2016
Gilbey V, Attrill MJ, Coleman RA (2008) Juvenile Chinese mitten crabs (Eriocheir sinensis) in the Thames estuary: distribution, movement and possible interactions with the native crab Carcinus maenas. Biol Invasions 10:67–77
Guan R (2000) Abundance and production of the introduced signal crayfish in a British lowland river. Aquac Int 8:59–76
Haddaway NR, Wilcox RH, Heptonstall REA et al (2012) Predatory functional response and prey choice identify predation differences between native/invasive and parasitised/unparasitised crayfish. PLoS ONE 7:e32229
Hänfling B, Edwards F, Gherardi F (2011) Invasive alien Crustacea: dispersal, establishment, impact and control. Biocontrol 56:573–595
Hansen GJA, Vander Zanden MJ, Blum MJ et al (2013) Commonly rare and rarely common: comparing population abundance of invasive and native aquatic species. PLoS ONE 8:e77415
Hatcher MJ, Dick JTA, Dunn AM (2014) Parasites that change predator or prey behaviour can have keystone effects on community composition. Biol Lett 10:20130879
Hirt MR, Lauermann T, Brose U, Noldus LPJJ, Dell AI (2017) The little things that run: a general scaling of invertebrate exploratory speed with body mass. Ecology 98:2751–2757
Holdich DM, Reynolds JD, Souty-Grosset C, Sibley PJ (2009) A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl Manag Aquat Ecosyst 394–395:11
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Holling CS (1964) The analysis of complex population processes. Can Entomol 96:335–347
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Hothorn T, Bretz F, Westfall P et al (2016) multcomp: simultaneous inference in general parametric models. R Package version 1.4.6. http://cran.r-project.org/package=multcomp. Accessed 12 Nov 2016
Jeschke JM, Kopp M, Tollrian R (2002) Predator functional responses: discriminating between handling and digesting prey. Ecol Monogr 72:95–112
Juliano SA (2001) Nonlinear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, Oxford, pp 178–196
Karatayev AY, Burlakova LE, Padilla DK et al (2009) Invaders are not a random selection of species. Biol Invasions 11:2009–2019
Kumschick S, Bacher S, Dawson W et al (2012) A conceptual framework for prioritization of invasive alien species for management according to their impact. NeoBiota 15:69–100
Lagos ME, White CR, Marshall DJ (2017) Do invasive species live faster? Mass-specific metabolic rate depends on growth form and invasion status. Funct Ecol 31:2080–2086
Larson ER, Twardochleb LA, Olden JD (2017) Comparison of trophic function between the globally invasive crayfishes Pacifastacus leniusculus and Procambarus clarkii. Limnology 18:275–286
Laverty C, Nentwig W, Dick JTA, Lucy FE (2015) Alien aquatics in Europe: assessing the relative environmental and socio- economic impacts of invasive aquatic macroinvertebrates and other taxa. Manag Biol Invasions 6:341–350
Lindqvist OV, Huner JV (1999) Life history characteristics of crayfish: what makes some of them good colonizers? In: Gherardi F, Holdich M (eds) Crayfish in Europe as alien species. How to make the best of a bad situation?. Balkema, Rotterdam, pp 23–30
Lodge DM, Kershner MW, Aloi JE, Covich AP (1994) Effects of an omnivorous crayfish (Orconectes rusticus) on a freshwater littoral food web. Ecology 75:1265–1281
Lohr CA, Hone J, Bone M et al (2017) Modeling dynamics of native and invasive species to guide prioritization of management actions. Ecosphere 8:e01822
Mathers KL, Chadd RP, Dunbar MJ et al (2016) The long-term effects of invasive signal crayfish (Pacifastacus leniusculus) on instream macroinvertebrate communities. Sci Total Environ 556:207–218
McFeeters BJ, Xenopoulos MA, Spooner DE et al (2011) Intraspecific mass-scaling of field metabolic rates of a freshwater crayfish varies with stream land cover. Ecosphere 2:art13
McKnight E, García-Berthou E, Srean P, Rius M (2017) Global meta-analysis of native and nonindigenous trophic traits in aquatic ecosystems. Glob Chang Biol 23:1861–1870
Médoc V, Spataro T, Arditi R (2013) Prey: predator ratio dependence in the functional response of a freshwater amphipod. Freshw Biol 58:858–865
Médoc V, Thuillier L, Spataro T (2018) Opportunistic omnivory impairs our ability to predict invasive species impacts from functional response comparisons. Biol Invasions. https://doi.org/10.1007/s10530-017-1628-5
Millidine KJ, Armstrong JD, Metcalfe NB (2009) Juvenile salmon with high standard metabolic rates have higher energy costs but can process meals faster. Proc R Soc B 276:2103–2108
Mills CD, Clark PF, Morritt D (2016) Flexible prey handling, preference and a novel capture technique in invasive, sub-adult Chinese mitten crabs. Hydrobiologia 773:135–147
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39:335–354
Murdoch A, Oaten WW (1975) Predation and population stability. Adv Ecol Res 9:1–131
Nilsson PA, Brönmark C (2000) Prey vulnerability to a gape-size limited predator: behavioural and morphological impacts on northern pike piscivory. Oikos 88:539–546
Nyström P, Brönmark C, Graneli W (1996) Patterns in benthic food webs: a role for omnivorous crayfish? Freshw Biol 36:631–646
Parker IM, Simberloff D, Lonsdale WM et al (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Pintor LM, Sih A, Kerby JL (2009) Behavioral correlations provide a mechanism for explaining high invader densities and increased impacts on native prey. Ecology 90:581–587
Pritchard DW, Paterson RA, Bovy HC, Barrios-O’Neill D (2017) FRAIR: an R package for fitting and comparing consumer functional responses. Methods Ecol Evol 8:1528–1534
Quetin LB (1983) Chapter II.5: an automated, intermittent flow respirometer for monitoring oxygen consumption and long-term activity of pelagic crustaceans. In: Gnaiger E, Forstner H (eds) Polarographic oxygen sensors: aquatic and physiological applications. Springer, Berlin, Heidelberg, pp 176–183
Rall BC, Brose U, Hartvig M et al (2012) Universal temperature and body-mass scaling of feeding rates. Philos Trans R Soc B Biol Sci 367:2923–2934
R Core Team (2016) R: a language and environment for statistical computing, version 3.3.1
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383
Rosewarne PJ, Mortimer RJG, Newton RJ et al (2016) Feeding behaviour, predatory functional responses and trophic interactions of the invasive Chinese mitten crab (Eriocheir sinensis) and signal crayfish (Pacifastacus leniusculus). Freshw Biol 61:426–443
Rudnick D, Resh V (2005) Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustacea. Freshw Biol 50:1323–1336
Rudnick DA, Hieb K, Grimmer KF, Resh VH (2003) Patterns and processes of biological invasion: the Chinese mitten crab in San Francisco Bay. Basic Appl Ecol 262:249–262
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Ann Rev Ecol Syst 35:305–332
Salo P, Korpimäki E, Banks PB et al (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc B 274:1237–1243
Sax DF, Gaines SD (2008) Species invasions and extinction: the future of native biodiversity on islands. Proc Natl Acad Sci USA 105:11490–11497
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502
Souty-Grosset C, Holdich DM, Noel PY, et al (2006) Atlas of crayfish in Europe. Muséum national d’Histoire naturelle, Paris (Patrimoines naturels 64)
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
Styrishave B, Bojsen BH, Witthøfft H, Andersen O (2007) Diurnal variations in physiology and behaviour of the noble crayfish Astacus astacus and the signal crayfish Pacifastacus leniusculus. Mar Freshw Behav Physiol 40:63–77
Sutherland WJ (1998) The importance of behavioural studies in conservation biology. Anim Behav 56:801–809
Svendsen MBS, Bushnell PG, Steffensen JF (2016) Design and setup of an intermittent-flow respirometry system for aquatic organisms. J Fish Biol 88:26–50
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Taylor NG (2016) Why are invaders invasive? Development of tools to understand the success and impact of invasive species. PhD Thesis, School of Biology, University of Leeds
Toscano BJ, Monaco CJ (2015) Testing for relationships between individual crab behavior and metabolic rate across ecological contexts. Behav Ecol Sociobiol 69:1343–1351
Twardochleb LA, Novak M, Moore JW (2012) Using the functional response of a consumer to predict biotic resistance to invasive prey. Ecol Appl 22:1162–1171
Twardochleb LA, Olden JD, Larson ER (2013) A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci 32:1367–1382
Weis JS (2010) The role of behavior in the success of invasive crustaceans. Mar Freshw Behav Physiol 43:83–98
Wellborn G, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Xu M, Mu X, Dick JTA et al (2016) Comparative functional responses predict the invasiveness and ecological impacts of alien herbivorous snails. PLoS ONE 11:e0147017
Yu H-X, Jiang C (2005) Effects of stocking Chinese mitten crab on the zoobenthos and aquatic vascular plant in the East Lake Reservoir, Heilongjiang, China. Acta Hydrobiol Sin 29:430–434
Acknowledgements
This work was carried out under a PhD Studentship 1299825 to NGT funded by the Natural Environment Research Council (NERC). AMD was supported by NERC Grant NE/G015201/1. We thank Rachel Paterson, David Aldridge, Keith Hamer, Lee Brown, Tom Doherty-Bone and Paula Rosewarne for advice, assistance or loan of equipment. We thank two anonymous reviewers for comments that improved the manuscript.
Author information
Authors and Affiliations
Contributions
NGT and AMD conceived and designed the experiments. NGT performed the experiments, analysed the data and wrote the manuscript. NGT and AMD edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Taylor, N.G., Dunn, A.M. Predatory impacts of alien decapod Crustacea are predicted by functional responses and explained by differences in metabolic rate. Biol Invasions 20, 2821–2837 (2018). https://doi.org/10.1007/s10530-018-1735-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1735-y