Abstract
Objectives
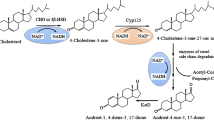
To enhance the yield of 9α-hydroxy-4-androstene-3,17-dione (9-OHAD) from phytosterols, a phytosterol transport system was constructed in Mycobacterium sp. strain MS136.
Results
9-OHAD can be produced via the controlled degradation of phytosterols by mycobacteria. This involves an active transport process that requires trans-membrane proteins and ATP. A phytosterol transport system from Mycobacterium tuberculosis H37Rv was constructed in Mycobacterium sp. strain MS136 by co-expression of an energy-related gene, mceG, and two integrated membrane protein genes, yrbE4A and yrbE4B. The resultant of the Mycobacterium sp. strain MS136-GAB gave 5.7 g 9-OHAD l−1, which was a 20% increase over 4.7 g l−1 by the wild-type strain. The yield of 9-OHAD was increased to 6.0 g l−1 by optimization of fermentation conditions, when 13 g phytosterols l−1 were fermented for 84 h in 30 ml biotransformation medium in shake flasks.
Conclusions
Phytosterol transport system plays an active role in the uptake and transport of sterols, cloning of the system improved the mass transfer of phytosterols and increased the production of 9-OHAD.




Similar content being viewed by others
References
Donova MV, Egorova OV (2012) Microbial steroid transformations: current state and prospects. Appl Microbiol Biotechnol 94:1423–1447
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzym Microbial Technol 32:688–705
Gao XQ (2016) Enhanced steroid metabolites production by resting cell phytosterol bioconversion. Chem Biochem Eng Q 29:567–573
Geize RVD, Yam K, Heuser T, Wilbrink MH, Hara H et al (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA 104:1947–1952
Joshi SM, Pandey AK, Capite N, Fortune SM, Rubin EJ, Sassetti CM (2006) Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci USA 103:11760–11765
Klepp LI, Forrellad MA, Osella AV, Blanco FC et al (2012) Impact of the deletion of the six mce operons in Mycobacterium smegmatis. Microbes Infect 14:590–599
Knol J, Bodewits K, Hessels GI, Dijkhuizen L, Geize RVD (2008) 3-Keto-5alpha-steroid delta(1)-dehydrogenase from Rhodococcus erythropolis SQ1 and its orthologue in Mycobacterium tuberculosis H37Rv are highly specific enzymes that function in cholesterol catabolism. Biochem J 410:339–346
Mohn WW, Geize RVD, Stewart GR, Okamoto S, Liu J, Dijkhuizen L, Eltis LD (2008) The actinobacterial mce4 locus encodes a steroid transporter. J Biol Chem 283:35368–35374
Pandey AK, Sassetti CM (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA 105:4376–4380
Wang Z, Zhao F, Chen D, Li D (2006) Biotransformation of phytosterol to produce androsta-diene-dione by resting cells of Mycobacterium in cloud point system. Process Biochem 41:557–561
Wei W, Wang FQ, Fan SY, Wei DZ (2010) Inactivation and augmentation of the primary 3-ketosteroid-Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microbiol 76:4578–4582
Wilbrink MH, Petrsma M, Dijkhuizen L, Geize RVD (2011) FadD19 of Rhodococcus rhodochrous DSM43269, a steroid-coenzyme a ligase essential for degradation of C-24 branched sterol side chains. Appl Environ Microbiol 77:4455–4464
Acknowledgements
The authors acknowledge the financial support by a startup funding from Tianjin University.
Supporting information
Supplementary Table 1—Strains, primers and plasmids used in this study.
Supplementary Fig. 1—HPLC analysis of 9-OHAD produced by engineered strains.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, K., Sun, H. & Song, H. Engineering phytosterol transport system in Mycobacterium sp. strain MS136 enhances production of 9α-hydroxy-4-androstene-3,17-dione. Biotechnol Lett 40, 673–678 (2018). https://doi.org/10.1007/s10529-018-2520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2520-9