Abstract
Objectives
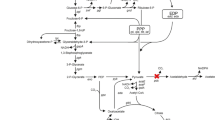
To investigate the roles of adhS, which encodes the AdhS subunit of membrane-bound alcohol dehydrogenase (mADH) in Gluconobacter oxydans DSM2003, and to rationally improve mADH activity.
Results
adhS was identified and overexpressed in G. oxydans DSM2003. Its overexpression promoted the AdhA subunit which serves as the primary dehydrogenase transfer from the periplasmic space to the periplasmic surface of the membrane thereby increasing the amount of active mADH and thus enhancing mADH activity up to 1.96-fold. The increased mADH activity significantly altered product selectivity (glyceric acid/dihydroxyacetone) during glycerol oxidation and increased the glyceric acid production by 7.6-fold. By comparison, overexpression of adhS and adhABS was equally effective in increasing the mADH activity and glyceric acid production.
Conclusions
adhS overexpression effectively improved mADH activity, indicating that for mADH, adhS might be a limiting component. The findings provide a guide for the efficient application of Gluconobacter spp. in hydroxy acid production.



Similar content being viewed by others
References
Claret C, Salmon J, Romieu C, Bories A (1994) Physiology of Gluconobacter oxydans during dihydroxyacetone production from glycerol. Appl Microbiol Biotechnol 41:359–365
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652
Habe H, Shimada Y, Yakushi T, Hattori H, Ano Y, Fukuoka T, Kitamoto D, Itagaki M, Watanabe K, Yanagishita H, Matsushita K, Sakaki K (2009) Microbial production of glyceric acid, an organic acid that can be mass produced from glycerol. Appl Environ Microbiol 75:7760–7766
Habe H, Fukuoka T, Morita T, Kitamoto D, Yakushi T, Matsushita K, Sakaki K (2010) Disruption of the membrane-bound alcohol dehydrogenase-encoding gene improved glycerol use and dihydroxyacetone productivity in Gluconobacter oxydans. Biosci Biotechnol Biochem 74:1391–1395
Inoue T, Sunagawa M, Mori A, Imai C, Fukuda M, Takagi M, Yano K (1989) Cloning and sequencing of the gene encoding the 72-kilodalton dehydrogenase subunit of alcohol dehydrogenase from Acetobacter aceti. J Bacteriol 171:3115–3122
Kondo K, Horinouchi S (1997) Characterization of the genes encoding the three-component membrane-bound alcohol dehydrogenase from Gluconobacter suboxydans and their expression in Acetobacter pasteurianus. Appl Environ Microbiol 63:1131–1138
Kondo K, Beppu T, Horinouchi S (1995) Cloning, sequencing, and characterization of the gene encoding the smallest subunit of the three-component membrane-bound alcohol dehydrogenase from Acetobacter pasteurianus. J Bacteriol 177:5048–5055
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Li MH, Wu J, Liu X, Lin JP, Wei DZ, Chen H (2010) Enhanced production of dihydroxyacetone from glycerol by overexpression of glycerol dehydrogenase in an alcohol dehydrogenase-deficient mutant of Gluconobacter oxydans. Bioresour Technol 101:8294–8299
Liebminger S, Hofbauer R, Siebenhofer M, Nyanhongo GS, Guebitz GM (2014) Microbial conversion of crude glycerol to dihydroxyacetone. Waste Biomass Valoriz 5:781–787
Masud U, Matsushita K, Theeragool G (2010) Cloning and functional analysis of adhS gene encoding quinoprotein alcohol dehydrogenase subunit III from Acetobacter pasteurianus SKU1108. Int J Food Microbiol 138:39–49
Prust C, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF, Ehrenreich A, Gottschalk G, Deppenmeier U (2005) Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechnol 23:195–200
Švitel J, Šturdík E (1994) Product yield and by-product formation in glycerol conversion to dihydroxyacetone by Gluconobacter oxydans. J Ferment Bioeng 78:351–355
Toyama H, Fujii A, Matsushita K, Shinagawa E, Ameyama M, Adachi O (1995) Three distinct quinoprotein alcohol dehydrogenases are expressed when Pseudomonas putida is grown on different alcohols. J Bacteriol 177:2442–2450
Wei G, Yang X, Gan T, Zhou W, Lin J, Wei D (2009) High cell density fermentation of Gluconobacter oxydans DSM 2003 for glycolic acid production. J Ind Microbiol Biotechnol 36:1029–1034
Wei L, Yang X, Gao K, Lin J, Yang S, Hua Q, Wei D (2010) Characterization of enzymes in the oxidation of 1,2-propanediol to D-(−)-lactic acid by Gluconobacter oxydans DSM 2003. Mol Biotechnol 46:26–33
Yakushi T, Matsushita K (2010) Alcohol dehydrogenase of acetic acid bacteria: structure, mode of action, and applications in biotechnology. Appl Microbiol Biotechnol 86:1257–1265
Acknowledgments
This work was supported by the National Key Basic Research Development Program of China (“973” Program; No. 2012CB721003), the Shanghai Natural Science Foundation (No. 15ZR1408600), the National Major Science and Technology Projects of China (No.2012ZX09304009), and the National High Technology Research and Development Program of China (“863” Program; No. 2012AA022201C).
Supporting Information
Supplementary Table 1—Primers used in this study.
Supplementary Figure 1—Nucleotide and amino acid sequences of adhS and upstream promoter in Gluconobacter oxydans DSM 2003.
Supplementary Figure 2—Comparison of amino acid sequences deduced from adhS in four Gluconobacter species.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Shi, L., Lin, J. et al. Effective improvement of the activity of membrane-bound alcohol dehydrogenase by overexpression of adhS in Gluconobacter oxydans . Biotechnol Lett 38, 1131–1138 (2016). https://doi.org/10.1007/s10529-016-2084-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2084-5