Abstract
Objectives
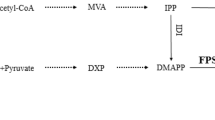
Farnesyl diphosphate synthase is a critical enzyme in the isoprenoids biosynthesis pathway responsible for ergosterol and secondary metabolites biosynthesis in fungi.
Results
Characterization of fds from Penicillium brevicompactum (Pbfds) was performed using TAIL-PCR and RT-PCR followed by complementation tests in Saccharomyces cerevisiae and determination of its expression profile by semi-quantitative RT-PCR. Promoter analysis suggests some binding sites for transcription factors some of which are involved in fungal growth and response to environmental stress. The Pbfds ORF encodes a cytosolic 39.7 kDa protein with a high conservation among Eurotiomycetes and the highest identity (96 %) with Pen. chrysogenum. Homology-based structural modeling suggests that the PbFDS is formed by the arrangement of 15 core helices around a large central cavity where the catalytic reaction takes place. Superimposition of the predicted 3D structure of the enzyme on its ortholog in human reveals the same folding pattern in the counterparts.
Conclusion
The Pbfds expression may be stimulated in response to the environmental stresses and fungal growth and encodes the PBFDS—a cytosolic enzyme which with a key role in ergosterol and secondary metabolites biosynthesis.







Similar content being viewed by others
References
Alcaíno J, Romero I, Niklitschek M, Sepúlveda D, Rojas MC, Baeza M, Cifuentes V (2014) Functional characterization of the Xanthophyllomyces dendrorhous farnesyl pyrophosphate synthase and geranylgeranyl pyrophosphate synthase encoding genes that are involved in the synthesis of isoprenoid precursors. PLoS One 9:e96626
Al-Samarrai TH, Schmid J (2000) A simple method for extraction of fungal genomic DNA. Lett Appl Microbiol 30:53–56
Coluccio AE, Rodriguez RK, Kernan MJ, Neiman AM (2008) The yeast spore wall enables spores to survive passage through the digestive tract of Drosophila. PLoS One 3:e2873
Dhar MK, Koul A, Kaul S (2013) Farnesyl pyrophosphate synthase: a key enzyme in isoprenoid biosynthetic pathway and potential molecular target for drug development. New Biotechnol 30:114–123
Ding Y-X, Ou-Yang X, Shang C-H, Ren A, Shi L, Li Y-X, Zhao M-W (2008) Molecular cloning characterization, and differential expression of a farnesyl-diphosphate synthase gene from the basidiomycetous fungus Ganoderma lucidum. Biosci Biotechnol Biochem 72:1571–1579
Joffrion TM, Cushion MT (2010) Sterol biosynthesis and sterol uptake in the fungal pathogen Pneumocystis carinii. FEMS Microbiol Lett 311:1–9
Kelley L, Sternberg M (2009) Protein structure prediction on the web: a case study using the Phyre server. Nat Protocol 4:363–371
Kim OT, Bang KH, Jung SJ, Kim YC, Hyun DY, Kim SH, Cha SW (2010) Molecular characterization of ginseng farnesyl diphosphate synthase gene and its up-regulation by methyl jasmonate. Biol Plant 54:47–53
MacPherson S, Larochelle M, Turcotte B (2006) A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev 70:583–604
Mekkriengkrai D, Sando T, Hirooka K, Sakdapipanich J, Tanaka Y, Fukusaki E-I, Kobayashi A (2004) Cloning and characterization of farnesyl diphosphate synthase from the rubber-producing mushroom Lactarius chrysorrheus. Biosci Biotechnol Biochem 68:2360–2368
Miziorko HM (2011) Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys 505(2):131–143
Park J, Lin Y-S, De Schutter JW, Tsantrizos YS, Berghuis AM (2012) Ternary complex structures of human farnesyl pyrophosphate synthase bound with a novel inhibitor and secondary ligands provide insights into the molecular details of the enzyme’s active site closure. BMC Struct Biol 12:32
Prokopenko V et al (2014) Design and synthesis of new potent inhibitors of farnesyl pyrophosphate synthase. Curr Drug Disc Technol 11:133–144
Romanelli MG, Lorenzi P, Sangalli A, Diani E, Mottes M (2009) Characterization and functional analysis of cis-acting elements of the human farnesyl diphosphate synthetase (FDPS) gene 5′-flanking region. Genomics 93:227–234
Singer T, Burke E (2003) High-throughput TAIL-PCR as a tool to identify DNA flanking insertions. Methods Mol Biol 236:241–271
Vishwakarma RK, Patel KA, Sonawane P, Singh S (2012) Molecular characterization of farnesyl pyrophosphate synthase from Bacopa monniera by comparative modeling and docking studies. Bioinformation 8:1075
Wang J, Li Y, Liu D (2014) Cloning and characterization of farnesyl diphosphate synthase gene involved in triterpenoids biosynthesis from Poria cocos. Int J Mol Sci 15:22188–22202
Wass MN, Kelley LA, Sternberg MJE (2010) 3D-LigandSite: predicting ligand-binding sites using similar structures. Nucl Acid Res 38:W469–W473
Yin H, Zhuang Y-B, E Li, H-P Bi, Zhou W, Liu T (2015) Heterologous biosynthesis of costunolide in Escherichia coli and yield improvement. Biotechnol Lett 37:1249–1255
Zeng Q, Qiu F, Yuan L (2008) Production of artemisinin by genetically-modified microbes. Biotechnol Lett 30:581–592
Acknowledgments
The authors would like to thank the National Institute of Genetic Engineering and Biotechnology (NIGEB) Foundation for supporting this work through Grant NIGEB-400.
Supporting information
Supplementary Table 1—Oligonucleotides used as primers.
Supplementary Table 2—PCR programs.
Supplementary Figure 1—Sequence of Pbfds gene in the genomic context.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharifirad, A., Mohammadian, S., Yakhchali, B. et al. Characterization of a farnesyl diphosphate synthase gene from Penicillium brevicompactum MUCL 19011. Biotechnol Lett 38, 71–79 (2016). https://doi.org/10.1007/s10529-015-1943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-1943-9