Abstract
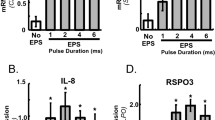
Skeletal muscle (SkM) is a tissue that responds to mechanical load following both physiological (exercise) or pathophysiological (bed rest) conditions. The heterogeneity of human samples and the experimental and ethical limitations of animal studies provide a rationale for the study of SkM plasticity in vitro. Many current in vitro approaches of mechanical loading of SkM disregard the three-dimensional (3D) structure in vivo. Tissue engineered 3D SkM, that displays highly aligned and differentiated myotubes, was used to investigate mechano-regulated gene transcription of genes implicated in hypertrophy/atrophy. Static loading (STL) and ramp loading (RPL) at 10 % strain for 60 min were used as mechano-stimulation with constructs sampled immediately for RNA extraction. STL increased IGF-I mRNA compared to both RPL and CON (control, p = 0.003 and 0.011 respectively) whilst MMP-9 mRNA increased in STL and RPL compared to CON (both p < 0.05). IGFBP-2 mRNA was differentially regulated in RPL and STL compared to CON (p = 0.057), whilst a reduction in IGFBP-5 mRNA was found for STL and RPL compared to CON (both p < 0.05). There was no effect in the expression of putative atrophic genes, myostatin, MuRF-1 and MAFBx (all p > 0.05). These data demonstrate a transcriptional signature associated with SkM hypertrophy within a tissue-engineered model that more greatly recapitulates the in vivo SkM structure compared previously published studies.






Similar content being viewed by others
References
Adams GR, Haddad F (1996) The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 81:2509–2516
Adams GR, McCue SA (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84:1716–1722
Adams GR, Haddad F, Baldwin KM (1999) Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol 87:1705–1712
Aguiar AF, Vechetti-Junior IJ, Alves de Souza RW, Castan EP, Milanezi-Aguiar RC, Padovani CR, Carvalho RF, Silva MD (2012) Myogenin, MyoD and IGF-I regulate muscle mass but not fiber-type conversion during resistance training in rats. Int J Sports Med 34:293–301
Armstrong RB, Marum P, Tullson P, Saubertt CW 4th (1979) Acute hypertrophic response of skeletal muscle to removal of synergists. J Appl Physiol Resp Environ Exerc Physiol 46:835–842
Auluck A, Mudera V, Hunt NP, Lewis MP (2005) A three-dimensional in vitro model system to study the adaptation of craniofacial skeletal muscle following mechanostimulation. Eur J Oral Sci 113:218–224
Awede B, Thissen J, Gailly P, Lebacq J (1999) Regulation of IGF-I, IGFBP-4 and IGFBP-5 gene expression by loading in mouse skeletal muscle. FEBS Lett 461:263–267
Awede BL, Thissen JP, Lebacq J (2002) Role of IGF-I and IGFBPs in the changes of mass and phenotype induced in rat soleus muscle by clenbuterol. Am J Physiol Endocrinol Metab 282:E31–E37
Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA (2003) Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol 94:2255–2262
Bickel CS, Slade J, Mahoney E, Haddad F, Dudley GA, Adams GR (2005) Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J Appl Physiol 98:482–488
Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C (1985) Plasticity of the differentiated state. Science 230:758–766
Carmeli E, Coleman R, Reznick AZ (2002) The biochemistry of aging muscle. Exp Gerontol 37:477–489
Caron MA, Charette SJ, Maltais F, Debigare R (2011) Variability of protein level and phosphorylation status caused by biopsy protocol design in human skeletal muscle analyses. BMC Res Notes 4:488
Carson JA, Nettleton D, Reecy JM (2002) Differential gene expression in the rat soleus muscle during early work overload-induced hypertrophy. FASEB J 16:207–209
Cheema U, Yang SY, Mudera V, Goldspink GG, Brown RA (2003) 3-D in vitro model of early skeletal muscle development. Cell Motil Cytoskelet 54:226–236
Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G (2005) Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol 202:67–75
Clemmons DR (1998) Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol 140:19–24
Coppock HA, White A, Aplin JD, Westwood M (2004) Matrix metalloprotease-3 and -9 proteolyze insulin-like growth factor-binding protein-1. Biol Reprod 71:438–443
Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M (2006) Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290:E731–E738
Dahiya S, Bhatnagar S, Hindi SM, Jiang C, Paul PK, Kuang S, Kumar A (2011) Elevated levels of active matrix metalloproteinase-9 cause hypertrophy in skeletal muscle of normal and dystrophin-deficient mdx mice. Hum Mol Genet 20:4345–4359
Degens H, Alway SE (2006) Control of muscle size during disuse, disease, and aging. Int J Sports Med 27:94–99
Eastwood M, McGrouther DA, Brown RA (1998a) Fibroblast responses to mechanical forces. Proc Inst Mech Eng H 212:85–92
Eastwood M, Mudera VC, McGrouther DA, Brown RA (1998b) Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil Cytoskelet 40:13–21
Esser KA, White TP (1995) Mechanical load affects growth and maturation of skeletal muscle grafts. J Appl Physiol 78:30–37
Friedmann-Bette B, Schwartz FR, Eckhardt H, Billeter R, Bonaterra G, Kinscherf R (2012) Similar changes of gene expression in human skeletal muscle after resistance exercise and multiple fine needle biopsies. J Appl Physiol 112:289–295
Glass DJ (2003) Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90
Goldberg AL (1967) Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol 213:1193–1198
Goldberg AL, Goodman HM (1969) Amino acid transport during work-induced growth of skeletal muscle. Am J Physiol 216:1111–1115
Goldspink DF (1977) The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol 264:267–282
Goldspink G (2005) Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda) 20:232–238
Goldspink DF, Cox VM, Smith SK, Eaves LA, Osbaldeston NJ, Lee DM, Mantle D (1995) Muscle growth in response to mechanical stimuli. Am J Physiol 268:E288–E297
Gumucio JP, Mendias CL (2012) Atrogin-1, MuRF-1, and sarcopenia. Endocrine 43(1):12–21
Henriksen EJ, Schneider MC, Ritter LS (1993) Regulation of contraction-stimulated system A amino acid uptake in skeletal muscle: role of vicinal sulfhydryls. Metabolism 42:440–445
Hubatsch DA, Jasmin BJ (1997) Mechanical stimulation increases expression of acetylcholinesterase in cultured myotubes. Am J Physiol 273:C2002–C2009
Iwata M, Hayakawa K, Murakami T, Naruse K, Kawakami K, Inoue-Miyazu M, Yuge L, Suzuki S (2007) Uniaxial cyclic stretch-stimulated glucose transport is mediated by a ca-dependent mechanism in cultured skeletal muscle cells. Pathobiology 74:159–168
James PL, Stewart CE, Rotwein P (1996) Insulin-like growth factor binding protein-5 modulates muscle differentiation through an insulin-like growth factor-dependent mechanism. J Cell Biol 133:683–693
Kandarian SC, Schulte LM, Esser KA (1992) Age effects on myosin subunit and biochemical alterations with skeletal muscle hypertrophy. J Appl Physiol 72:1934–1939
Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM (2005) Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol 99:2149–2158
Lenk K, Schuler G, Adams V (2010) Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachex Sarcopenia Muscle 1:9–21
Lewis MP, Tippett HL, Sinanan AC, Morgan MJ, Hunt NP (2000) Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J Muscle Res Cell Motil 21:223–233
Linderman JK, Talmadge RJ, Gosselink KL, Tri PN, Roy RR, Grindeland RE (1996) Synergistic ablation does not affect atrophy or altered myosin heavy chain expression in the non-weight bearing soleus muscle. Life Sci 59:789–795
Liu X, Lee DJ, Skittone LK, Natsuhara K, Kim HT (2010) Role of gelatinases in disuse-induced skeletal muscle atrophy. Muscle Nerve 41:174–178
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103:1744–1751
Martin NR, Passey SL, Player DJ, Khodabukus A, Ferguson RA, Sharples AP, Mudera V, Baar K, Lewis MP (2013) Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials 34:5759–5765
Matheny RW, Merritt E, Zannikos SV, Farrar RP, Adamo ML (2009) Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp Biol Med 234:164–170
McCarthy JJ, Esser KA (2007) Counterpoint: satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1100–1103
McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G (1999) Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516(Pt 2):583–592
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Mehan RS, Greybeck BJ, Emmons K, Byrnes WC, Allen DL (2011) Matrix metalloproteinase-9 deficiency results in decreased fiber cross-sectional area and alters fiber type distribution in mouse hindlimb skeletal muscle. Cells Tissues Organs 194:510–520
Mudera VC, Pleass R, Eastwood M, Tarnuzzer R, Schultz G, Khaw P, McGrouther DA, Brown RA (2000) Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil Cytoskelet 45:1–9
Mudera V, Smith AS, Brady MA, Lewis MP (2010) The effect of cell density on the maturation and contractile ability of muscle derived cells in a 3D tissue-engineered skeletal muscle model and determination of the cellular and mechanical stimuli required for the synthesis of a postural phenotype. J Cell Physiol 225:646–653
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Narici MV, Maffulli N (2010) Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95:139–159
O’Connor RS, Pavlath GK (2007) Point:Counterpoint: satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1099–1100
Passey S, Martin N, Player D, Lewis MP (2011) Stretching skeletal muscle in vitro: does it replicate in vivo physiology? Biotechnol Lett 33(8):1513–1521
Pillard F, Laoudj-Chenivesse D, Carnac G, Mercier J, Rami J, Riviere D, Rolland Y (2011) Physical activity and sarcopenia. Clin Geriatr Med 27:449–470
Powell CA, Smiley BL, Mills J, Vandenburgh HH (2002) Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 283:C1557–C1565
Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM (2003) Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc 51:636–641
Rehfeldt C, Renne U, Sawitzky M, Binder G, Hoeflich A (2010) Increased fat mass, decreased myofiber size, and a shift to glycolytic muscle metabolism in adolescent male transgenic mice overexpressing IGFBP-2. Am J Physiol Endocrinol Metab 299:E287–E298
Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, Heino J (1995) Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem 270:13548–13552
Rosenblatt JD, Parry DJ (1992) Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol 73:2538–2543
Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA (2003) Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 228:706–709
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology 23:160–170
Schiaffino S, Bormioli SP, Aloisi M (1972) Cell proliferation in rat skeletal muscle during early stages of compensatory hypertrophy. Virchows Arch B Cell Pathol 11:268–273
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Severgnini S, Lowenthal DT, Millard WJ, Simmen FA, Pollock BH, Borst SE (1999) Altered IGF-I and IGFBPs in senescent male and female rats. J Gerontol A Biol Sci Med Sci 54:B111–B115
Sharples AP, Stewart CE (2011) Myoblast models of skeletal muscle hypertrophy and atrophy. Curr Opin Clin Nutr Metab Care 14:230–236
Sharples AP, Al-Shanti N, Stewart CE (2010) C2 and C2C12 murine skeletal myoblast models of atrophic and hypertrophic potential: relevance to disease and ageing? J Cell Physiol 225:240–250
Sharples AP, Player DJ, Martin NR, Mudera V, Stewart CE, Lewis MP (2012) Modelling in vivo skeletal muscle ageing in vitro using three-dimensional bioengineered constructs. Aging Cell 11:986–995
Sharples AP, Al-Shanti N, Hughes DC, Lewis MP, Stewart CE (2013) The role of insulin-like-growth factor binding protein 2 (IGFBP2) and phosphatase and tensin homologue (PTEN) in the regulation of myoblast differentiation and hypertrophy. Growth Horm IGF Res 23:53–61
Smith AS, Passey S, Greensmith L, Mudera V, Lewis MP (2012) Characterization and optimization of a simple, repeatable system for the long term in vitro culture of aligned myotubes in 3D. J Cell Biochem 113:1044–1053
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6 K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:C1258–C1270
van den Beld AW, Blum WF, Pols HA, Grobbee DE, Lamberts SW (2003) Serum insulin-like growth factor binding protein-2 levels as an indicator of functional ability in elderly men. Eur J Endocrinol 148:627–634
Vandenburgh HH (1988) A computerized mechanical cell stimulator for tissue culture: effects on skeletal muscle organogenesis. In Vitro Cell Dev Biol 24:609–619
Vandenburgh H, Kaufman S (1979) In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203:265–268
Wang Q, McPherron AC (2012) Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J Physiol 590:2151–2165
Welle SL (2009) Myostatin and muscle fiber size. Focus on “Smad2 and 3 transcription factors control muscle mass in adulthood” and “Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size”. Am J Physiol Cell Physiol 296:C1245–C1247
Wolf E, Schneider MR, Zhou R, Fisch TM, Herbach N, Dahlhoff M, Wanke R, Hoeflich A (2005) Functional consequences of IGFBP excess-lessons from transgenic mice. Pediatr Nephrol 20:269–278
Yang Y, Jemiolo B, Trappe S (2006) Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol 101:1442–1450
Zanchi NE, de Siqueira Filho MA, Lira FS, Rosa JC, Yamashita AS, de Oliveira Carvalho CR, Seelaender M, Lancha-Jr AH (2009) Chronic resistance training decreases MuRF-1 and Atrogin-1 gene expression but does not modify Akt, GSK-3beta and p70S6K levels in rats. Eur J Appl Physiol 106:415–423
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Player, D.J., Martin, N.R.W., Passey, S.L. et al. Acute mechanical overload increases IGF-I and MMP-9 mRNA in 3D tissue-engineered skeletal muscle. Biotechnol Lett 36, 1113–1124 (2014). https://doi.org/10.1007/s10529-014-1464-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1464-y