Abstract
Skeletal muscle is the most abundant tissue in the human body, and the maintenance of its mass is essential to ensure basic function as locomotion, strength and respiration. The decision to synthesize or to break down skeletal muscle proteins is regulated by a network of signaling pathways that transmit external stimuli to intracellular factors regulating gene transcription. The tightly regulated balance of muscle protein breakdown and synthesis is disturbed in several distinct myopathies, but also in two pathologies: sarcopenia and cachexia. In recent years, it became evident that in these two muscle wasting disorders specific regulating molecules are increased in expression (e.g. members of the ubiquitin–proteasome system, myostatin, apoptosis inducing factors), whereas other factors (e.g. insulin-like growth factor 1) are down-regulated. So far, not many treatment options to fight the muscle loss are available. One of the most promising approaches is exercise training that, due to its multifactorial effects, can act on several signaling pathways. Therefore, this review will concentrate on specific alterations discussed in the current literature that are present in the skeletal muscle of both muscle wasting disorders. In addition, we will focus on exercise training as an intervention strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Skeletal muscle comprises a system that represents the largest organ of the human body. As it consists of over 600 separate muscles it is the major reservoir of body proteins and takes up to 50% of the total body weight. Its main functions are not only to provide movement, strength and respiration but also to balance posture and to regulate the body temperature. Besides distinct myopathies there are two pathologies of muscle wasting which are of general interest, namely sarcopenia and cachexia.
Sarcopenia, meaning poverty of flesh, is the degenerative unintentional loss of skeletal muscle mass and strength associated with aging [1]. It is estimated that the prevalence of sarcopenia in community-dwelling older adults is approximately 25% [2], and there is a loss of 5% of muscle mass per decade of life from the fourth decade onwards, potentially increasing after the age of 65 years [3, 4]. From a histological point of view, sarcopenia is characterized by a decrease in the number and the size of the muscle fibers.
Cachexia is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass [5]. Cachexia typically manifests in chronic diseases such as cancer, chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF) and chronic kidney disease [6] (CKD) (Fig. 1). The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). The key component was at least a 5% loss of edema-free body weight during the previous 12 months or less.
A major barrier to effective management of skeletal muscle wasting is the inadequate understanding of its underlying biological mechanisms. Therefore, in this review, we will concentrate on specific alterations discussed in the current literature that are present in the skeletal muscle of both muscle wasting disorders. In addition, we will focus on exercise training as a strategy to intervene with these alterations.
2 Protein catabolism/anabolism—ubiquitin proteasome system and autophagy
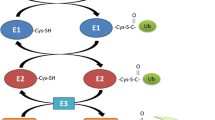
The most evident metabolic explanation for muscle decline is an imbalance between protein catabolism and anabolism. At least four major proteolytic pathways (lysosomal, Ca2+-dependent, caspase-dependent and ubiquitin–proteasome-dependent) operate in skeletal muscle, and may be altered in the process of sarcopenia and muscle cachexia. Aside from these four distinct pathways, the autophagic/lysosomal pathway also has to be considered. In this pathway, portions of the cytoplasm and cell organelles are sequestered into autophagosomes, which subsequently fuse with lysosomes, where the proteins are digested [7]. Dissecting the molecular regulation of the ubiquitin–proteasome-dependent system (UPS) and autophagy it became evident that forkhead box O (FoxO) transcription factors take a central position. FoxO transcription factors, normally phosphorylated and inactivated by PI3K-Akt/PKB, translocate into the cell nucleus and induce the transcription of the skeletal muscle-specific E3 ubiquitin ligases, MuRF1 and MAFbx/atrogin [8], as well as autophagy-related genes like LC3 and Bnip3 [9]. Upstream of PI3K-Akt, several factors like reactive oxygen species (ROS), tumor necrosis factor α (TNF-α), the tumor-released proteolysis-inducing factor (PIF), the peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1α) or insulin-like growth factor 1 (IGF-1) have been shown to influence this regulatory system [8, 10–12].
On the other hand, protein anabolic factors like IGF-1 are counteracting muscle atrophy. Besides inhibiting autophagy and the UPS, IGF-1 activates via Akt–mTOR (mammalian target of rapamycin)–p70S6K (p70 S6 kinase) protein synthesis [13, 14].
2.1 Sarcopenia
Experimental and human studies in the last decade clearly demonstrated that UPS is activated in several muscle wasting conditions (reviewed by Mitch and Goldberg [15]). However, data on muscle wasting by the ubiquitin–proteasome system (UPS) in aging are conflicting. Several authors described an up-regulation of components of the UPS in sarcopenia [16–18], whereas others found a down-regulation [19–21] or no change [22]. Therefore, at least in sarcopenia, the UPS seems not to be the major pathway responsible for muscle loss.
Calpains belong to a large family of calcium-dependent cystein proteases, and demonstrate a ubiquitous or tissue-specific expression [23]. Besides its regulation by calcium, calpain activity is tightly controlled by its inhibitor calpastatin [24]. In an animal study comparing the mRNA expression of calpains and calpastatin in the skeletal muscle of 3- and 24-month-old rats, a 38% increase in μ-calpain and a 28% decrease in calpastatin in the old specimens was evident [25]. In addition, these changes at the expression levels were confirmed by calpain activity measurements. At least, these animal data point to a possible involvement of the calpains in muscle loss during ageing. Nevertheless, this has to be confirmed in human muscle biopsies, and further experiments have to elucidate the physiological targets of calpains in sarcopenia.
Lysosomes are responsible for the degradation of long-lived proteins and for the enhanced protein degradation observed under starvation conditions. Using a gene expression profile analysis from young (3–4 months) and old (30–31 months) rats, Pattison and colleagues [26] described a slight up-regulation of cathepsin L in the old soleus muscle. Nevertheless, this result could not be confirmed in a later study by O’Connell et al. [27], who screened for differentially expressed protein in the gastrocnemius muscle of 30- and 3-month-old rats. Data for the direct analysis of lysosomal components in young vs. old skeletal muscle are missing.
At least in transgenic mice overexpressing IGF-1 specifically in the skeletal muscle, it was evident that the age-related sarcopenia was prevented [28]. Furthermore, it is well known that post-maturational aging is associated with reduced serum IGF-1 concentration. This finding was supported by detecting a reduced expression level in the skeletal muscle of older men when compared to younger ones [29, 30]. Unfortunately, no correlation between muscle mass or protein synthesis rate was found [30], whereby the functional significance of the alterations are uncertain.
2.2 Cachexia
The UPS is the major proteolytic machinery systematically activated in cachexia. To assess the role of the UPS in cancer cachexia, Williams and colleagues [31] took biopsies of cancer and non-cancer patients undergoing laparotomy for various reasons. The mRNA levels for ubiquitin and the 20 S proteasome subunits were two to four times higher in muscle from patients with cancer than in muscle from control patients.
In a report by Lecker et al. [32], muscles atrophying from different causes (cancer cachexia, streptozotocin-induced diabetes mellitus, uremia induced by subtotal nephrectomy, and from pair-fed control rats) were investigated. Proteins involved in protein degradation, including polyubiquitins, Ub fusion proteins, the Ub ligases atrogin-1/MAFbx and MuRF-1, multiple but not all subunits of the 20 S proteasome and its 19 S regulator, and cathepsin L were up-regulated [32].
In a cancer cachexia animal study by Acharyya and coworkers [33] it was demonstrated that myosin heavy chain (MyHC) is a selective target associated with a wasting state compared to other myofibrillar proteins [33]. MyHC protein was significantly reduced, whereas MyHC mRNA levels were unchanged. Results showed that the mature form of MyHC could readily be immunoprecipitated with ubiquitin, which supports the involvement of this proteolytic pathway in the basal turnover of myosin.
The important role of the FoxO transcription factors was underlined in a study by Liu et al. [34] when they targeted Foxo-1 in a cancer cachexia mice model by an oligonucleotide. It could be demonstrated that the RNA oligonucleotide can reduce the expression of Foxo-1 in normal and cachectic mice, leading to an increase in skeletal muscle mass of the mice. In the search for downstream target genes of Foxo-1, increased levels of MyoD and decreased concentrations of myostatin were found.
Investigating the UPS and the lysosomal proteolytic pathway in lung cancer patients, Jagoe and colleagues reported that mRNA levels for cathepsin B, but not for components of the ubiquitin–proteasome pathway, were higher in patients with cancer compared with controls suggesting that cathepsin B may have a role in inducing muscle wasting in the early stages of lung cancer [35].
Besides an increased catabolism, there is reduced anabolism, which has been shown, for example in cancer-related cachexia. Although the underlying mechanism remains unknown, it could be demonstrated that the IGF-1 system is down-regulated in an animal model of cancer cachexia [36]. Interestingly, the transgenic overexpression of locally acting IGF-1 in skeletal muscle inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction [37].
In cancer cachexia, in particular, the decrease in skeletal muscle protein synthesis is partly related to the increased serum level of the PIF. Intravenous administration of PIF to normal mice produced a rapid decrease in body weight that was accompanied by increased mRNA levels for ubiquitin in the gastrocnemius muscle [11]. There were also increased protein levels of the 20 S proteasome core and 19 S regulatory subunit, suggesting activation of the ATP–ubiquitin-dependent proteolytic pathway. Recent evidence proposes that PIF decreases protein synthesis by inhibiting protein translation initiation through phosphorylation of the eukaryotic initiation factor 2 (eIF2-alpha) [38].
Another factor that may contribute to a decreased anabolism is angiotensin II. In an animal model of continuously administered angiotensin II, a markedly reduced plasma IGF I levels occurred [39]. Compared with sham, angiotensin II-infused hypertensive rats lost 18–26% of body weight by 1 week, which was completely reversible by losartan, an AT1 receptor antagonist.
3 Oxidative stress—ROS
Oxidative stress is a state wherein the normally well-balanced control of oxidant production and antioxidant capacity is disturbed. The sources of oxidants are numerous and include enzymatic and chemical reactions producing superoxide anions, hydrogen peroxides or nitric oxide. Once produced, these molecules can interact with each other to form even more highly reactive products like peroxynitrite (ONOO-) or hydroxyl radicals (OH.). At basal levels these molecules fulfill important signaling tasks, but when the concentration rises above a certain level detrimental effects like alterations of lipids, proteins and even DNA are overwhelming. There is plenty of evidence in the current literature that oxidative stress is associated with chronic diseases, and it is assumed that an increase in ROS directs muscle cells into a catabolic state leading to muscle wasting [40–42]. At least from cell culture experiments mainly performed in C2C12 cells, it is well documented that ROS have the potency to induce the expression of E3-ubiquitin ligases [43], correlating with an increased ubiquitin-conjugating activity and proteasome activity and decreased myosin protein [43, 44]. In addition, oxidative stress is a potent inducer of apoptotic cell death [45].
3.1 Sarcopenia
The free radical theory of aging formulated more than 50 years ago proposes that aging and associated degenerative diseases can be attributed to the effect of ROS [46]. A current version of this theory is the oxidative stress theory of aging, stating that a chronic state of oxidative stress even exists under normal conditions and is increased with aging due to an imbalance of generation and detoxification of ROS [47]. An elegant method to estimate the load of oxidative stress is the measurement of protein modifications, like carbonylation or nitration. Using this approach, Feng and coworkers could demonstrate a significant up-regulation of carbonylated proteins of mitochondria from older (26 months) vs. younger (12 months) Fisher 344 rats [48]. This is further supported by several studies linking the amount of carbonylated protein with muscle strength [49]. The other marker for increased oxidative stress is 3-nitrotyrosine (3-NT). This posttranslational modification can alter protein function and activity, and is formed when tyrosine residues are nitrated by peroxynitrite [50]. A case-control study measuring 3-NT immunoreactivity in external intercostals and quadriceps muscle obtained from elderly and young individuals revealed a significant increase in the elderly [51]. In animal studies, 3-NT was evaluated in muscles from young, adult and old rats [52–54]. A significant age-associated increase in nitrotyrosine-modified proteins involved in metabolism, contraction and calcium homeostasis was observed. This increase of oxidative stress in the aged skeletal muscle may be due to a reduction in the detoxification system due to a lower expression/activity of Mn-SOD or catalase [51, 55].
3.2 Cachexia
Also, in cachexia, ROS are regarded as crucial players for muscle protein catabolism by stimulating the UPS. As mentioned above, reaction products are measured as indirect markers for oxidative stress. In cachexia, malondialdehyde (MDA) is regarded as such indirect marker. In an animal study, a significantly increased level of MDA in muscles from cachectic MAC16 mice was detected, when compared with control non-tumor-bearing animals [44]. In addition, experimental cancer cachexia appears to be mediated by increased nitrosative stress secondary to increased nitric oxide formation. Indeed, protein tyrosine nitration is markedly increased in the muscles of tumor bearing rats with advanced cachexia [56]. Also, in cachexia, this increase in ROS is due to significantly lower activities of antioxidant enzymes: superoxide dismutase and glutathione peroxidase [57].
4 Inflammation
Since the influential report of elevated levels of TNF-α in patients with cardiac cachexia, it has become clear that pro-inflammatory cytokines play an important role in the evolvement of cachexia and other muscle wasting disorders [58]. TNF-α either on its own or in combination with other cytokines can induce the breakdown of mature myotubes [59, 60]. For example TNF-α and IFN-γ act synergistically not only to inhibit the activation of messenger RNA for MyHC synthesis, but also for stimulating the proteolysis of this protein [33]. With respect to the UPS, cell culture studies revealed that TNF-α in particular is a potent stimulator of MuRF1 and MAFbx expression [61, 62]. Based on animal experiments, it even seems that MuRF1 is essential for TNF-α-induced loss of muscle function [63].
Exposure of myocytes to TNF-α rapidly activates the transcription factor NF-κB, which in turn inhibits muscle cell differentiation by suppressing the synthesis of MyoD, thereby influencing muscle regeneration (see below). It should be noted that the activation of NF-kB is also involved in the up-regulation of cytokine synthesis, which can contribute to paracrine effects of cytokines on skeletal muscle tissue as described above.
Thus, cytokine-induced skeletal muscle wasting is probably a multifactorial process, involving increased protein degradation and reduced myocyte regeneration and repair [64].
4.1 Sarcopenia
There is growing evidence that higher levels of inflammatory markers are associated with physical decline in older persons, possibly through the catabolic effects of inflammatory markers on muscle. In an observational study of more than 2,000 men and women, TNF-α showed a consistent association with the decline in muscle mass and strength [65]. The impact of inflammation in the development of sarcopenia is furthermore supported by a recently published animal study showing that reduction of low-grade inflammation by ibuprofen in old (20 months) animals resulted in a significant decreased muscle mass loss [66]. The loss of muscle mass in the situation of low-grade inflammation is possibly due to a loss of stimulation in protein synthesis by food intake with an unaltered rate of protein degradation [67].
4.2 Cachexia
TNF-α, interleukin-1 (IL-1), IL-6 and IFN-γ have been implicated in the induction of cancer-related muscle wasting [68]. There is growing evidence that the accelerated muscle proteolysis during malignant tumor growth is mediated via the activation of the non-lysosomal adenosine triphosphate-dependent (ATP-dependent) ubiquitin proteasome pathway [31, 69]. In addition, inflammatory cytokines influence the expression of functionally relevant enzymes in cardiac cachexia. It has been demonstrated that TNF-α, IFN-γ and IL1-β, which are known to be increased in cachectic patients, are potent activators of inducible nitric oxide synthase (iNOS) expression [70], which in turn produces toxic levels of NO high enough to inhibit key enzymes of the oxidative phosphorylation. It could be shown in vitro that NO is able to impair the contractile performance of the skeletal muscle [71].
5 Regenerative capacity and cell death
Adult muscle contains a pool of undifferentiated cells located between the basal lamina and the plasma membrane with the capacity to proliferate and differentiate only when required. These cells were first identified in the frog skeletal muscle and designated ‘satellite cells’ [72]. They are responsible for pre- and postnatal muscle growth, and are capable of repairing skeletal muscle fibers following injury. The proliferative life span of human satellite cells is limited with the potential number of cell divisions decreasing considerably as a function of donor age [73]. The differentiation of myogenic cells is under the control of the MyoD family of transcription factors, including MyoD, myogenin, Myf5 and MRF4 [74]. In the quiescent state of the satellite cells MyoD expression is absent, but as soon as they are activated by external stimuli they start to proliferate with high levels of MyoD in their nuclei [75]. Using yeast two-hybrid screens, an interaction between MAFbx and MyoD was evident, suggesting that MyoD is ubiquitinated by the atrophy-related E3 ubiquitin ligase MAFbx and subsequently degraded by the proteasome system [76].
Another factor regulating the regenerative capacity of the skeletal muscle is myostatin, a member of the transforming growth factor (TGF-β) superfamily. This relation was first evident in myostatin knockout mice, exhibiting an improved healing after a myotoxic injury [77]. Dissecting the molecular pathway, it became evident that myostatin influences satellite cell proliferation and differentiation through the down-regulation of Pax7 [78], an important factor of satellite cell proliferation [79]. Besides the regulation of satellite cell differentiation via MyoD, increased oxidative stress also impairs the regenerative capacity of these cells [80]. Therefore, in the case of muscle atrophy with an up-regulation of MuRF1/MAFbx and an elevated oxidative stress, the regenerative capacity of satellite cells is also impaired.
Regarding muscle atrophy, the loss of skeletal muscle myocytes via the energy-dependent programmed cell death apoptosis also needs to be considered. Apoptosis is well known to play an important physiological role during embryogenic development and in the control of cell number in proliferative tissue. A central component of the signal mechanism leading to apoptotic cell death is the activation of a series of caspases [81]. In particular, the activation of caspase-3 seems to be an initial step triggering accelerated muscle proteolysis in catabolic conditions [82, 83]. Sequentially, the activation of caspase-3, potentially regulated via PI3K [83], disassociates actomyosin complexes as a rate-limiting step, before the UPS can degrade the contractile proteins of the muscle [83].
5.1 Sarcopenia
Studies investigating the age-related concentration of satellite cells have produced diverging results. In one of the first studies performed, Snow [84] reported a decrease in satellite cells from 4.6% at 8 months of age to 2.4% at 30 months. Subsequent studies have either confirmed this result [85, 86] or have found no change with aging [87, 88]. Based on these discrepant results, it was suggested to focus more on function instead of the pure amount of the satellite cells in a given muscle. Analyzing the activation of satellite cells by nitric oxide or stretch in cell culture obtained from mice at different age revealed that satellite cells are increasingly refractory to an activation in aged mouse-muscle cultures [89]. An increase in oxidative stress, due to an imbalance in the antioxidative system may be partially responsible for the age-dependent decline in satellite function [90]. As discussed above, myostatin is an important factor regulating satellite cell proliferation and differentiation. Comparing the results published in the current literature, the relevance of myostatin for age-related muscle atrophy is unclear. Some authors describe no age-related change in myostatin expression, whereas others describe a significant up- or even down-regulation at the mRNA and/or protein level [30, 91–94]. With respect to the association of apoptosis/apoptosis related proteins and sarcopenia, the data currently available are more solid. An often used animal model to investigate sarcopenia is the Fischer 344 Brown Norway rat. Using either the release of mitochondrial cytochrom c [95], or TUNEL staining [96], or caspase- and caspase-9 activity [96, 97], or DNA fragmentation [97, 98] as marker for apoptosis, apoptosis was significantly increased in older animals when compared to younger rats.
At least for sarcopenia, the involvement of skeletal apoptosis seems to be proven, whereas the participation of satellite cells and myostatin needs further investigations.
5.2 Cachexia
As pointed out above, experimental data suggest that local IGF-1 may act as a regenerative agent, promoting recruitment of stem cells to sites of muscle injury [99]. As IGF-1 is reduced in experimental cachexia [36], it is reasonable to assume that in the cachectic situation the function of satellite cells is impaired. Other factors controlling the differentiation of satellite cells to functional fibers are nuclear factor kappa B (NFκB) and myostatin. Data are available demonstrating a beneficial effect of myostatin inhibition in cancer cachexia [100], but negative study results are also reported [101].
With respect to apoptosis, several reports demonstrated an increase in apoptosis or apoptosis-related protein in skeletal muscle after induction of cachexia. The skeletal muscle of cachectic tumor-bearing animals reveals the activation of DNA fragmentation, a hallmark of apoptosis [102]. These results from animal experiments could be confirmed in muscle biopsies from weight-losing patients with upper gastrointestinal cancer, where a significant increase in muscle DNA fragmentation (threefold) was documented [103]. In addition to DNA fragmentation, a significant up-regulation of caspase-1, -3, -6, -8, and -9 activity could also be documented in the gastrocnemius muscle of tumor-bearing mice [104].
Taken together, it seems that in cachexia and sarcopenia similar mechanisms are activated or deactivated to shift the balance towards protein breakdown, finally leading to a loss of muscle mass (Fig. 2a).
6 Anabolic steroids
Testosterone and testosterone derivatives are steroid hormones that exert their effects through binding to cytosolic receptors, which leads to an increase in protein synthesis and muscle mass [105]. Testosterone effects on skeletal muscle mass are dose-dependent, with administration of supraphysiological doses leading to a substantial increase in muscle size and strength. Interestingly, studies determining the effects of testosterone on muscle performance showed that testosterone administration is associated with an increase in leg power and strength but showed no change in muscle fatigability and no change in specific tension, indicating that testosterone-induced gains in muscle strength are reflective of an increase in muscle mass [106]. The increase in muscle mass is hypertrophic growth, as it is associated with an increase in myofiber cross-sectional area, observed both in type I and type II myofibers [107], and is not due to an increase in the number of myofibers. In addition, no change is observed in the number of fibers per unit of muscle; however, an increase in myonuclear number is apparent and is hypothesized to be attributable to fusion with satellite cells [107].
6.1 Sarcopenia
Among the hormonal changes that might be related to aging, a primary role is likely exerted by the aging-related deficit of anabolic hormones, promoting a milieu that favours catabolism. This aging-related deficiency in anabolic hormone milieu takes several different forms, being relatively sudden and dramatic in the case of estrogen (E2) in women, although being more gradual and steady for testosterone (Te) in men, and dihydroepiandosterone (DHEA) and growth hormone in both genders.
Numerous studies have been performed during the past years with different outcomes. Some have reported modest increases in lean mass [108, 109]. Some have reported increased grip strength [110, 111] and others did not [109, 112, 113]. Several studies have addressed the question of whether testosterone replacement increases lower body strength [108–110, 113–115]. with only two obtaining substantial positive results [115, 116]. Nevertheless, the magnitude of strength increases, although substantial, is lower than what can be achieved through resistance exercise training.
6.2 Cachexia
There is a relative deficiency or resistance to anabolic hormones in cachectic states. Up to 50% of men with metastatic cancer prior to chemotherapy can present with low concentrations of testosterone [117]. A reduction in testosterone might lead to a reduced bone mass, muscle strength and sexual function in both men and women [118, 119]. Based on these and several other observations, Evans and colleagues [5] have suggested a new definition and pathophysiology for cachexia. Low concentrations of testosterone and other anabolic hormones are major contributors to cachexia related wasting of skeletal muscle [5]. However, with respect to a correlation of body composition including muscle mass and the concentration conflicting results were reported in the current literature. Some studies found a correlation [117, 120], whereas others reported no association [121].
7 Impact of exercise training
Although it is increasingly recognized that exercise training seems to be a poly-pill against the dramatic changes in the skeletal muscle in cachexia, scientific proof is scarce. As a matter of fact, there are only very few clinical trials investigating the impact of exercise training in cachexia. A few small studies have shown that exercise training leads to changes in body composition. Investigations with larger cohorts and hard end points are still missing. Most of the research concerning exercise training and cachexia has been done in the field of cancer cachexia, preferably with animal models. Therefore, the majority of data covers this entity. However, as there are numerous similarities in the pathophysiology of cachexia regardless of its origin, these facts might be applicable to other facets of this syndrome.
With respect to sarcopenia, exercise training is the most effective and safe intervention to attenuate or recover some of the loss of muscle mass and strength that accompanies aging (reviewed by Jones et al. [122]).
7.1 Mode of exercise training and obvious effects
There are mainly two different types of exercise training: endurance and resistance exercise training. Since the early 1980 s, nurse researchers and others have shown that endurance exercise has beneficial effects in cancer patients. Several reports propose that endurance training can ameliorate cancer related fatigue. In various studies, self-reported fatigue could be significantly reduced by different types of endurance training [123, 124]. Furthermore, it could be demonstrated that endurance training led to an improved physical capacity in cancer patients [123, 125]. Additional evidence for the beneficial role of endurance exercise has been provided by data generated from animal studies of experimental tumor-bearing rodents [126, 127]. Endurance training such as running, treadmill walking, and swimming have been associated with smaller tumors and greater food consumption. Endurance exercise was associated with an increase in muscle protein synthesis and in muscle to body weight ratio. It should be emphasized, however, that the exercised animals had smaller tumors and consumed more food compared to the sedentary tumor-bearing animals. Therefore, it is not clear whether the observed positive change in muscle protein metabolism was a consequence of smaller tumors and higher food intake or a consequence of a direct effect of the exercise on the muscles. Besides these animal studies, four studies examined the effects of resistance training on skeletal muscle mass and/or strength in patients with cancer. Two studies investigated the effect of resistance training in breast cancer patients receiving adjuvant therapy [128, 129]. Women in the resistance training group had a significant increase in lean muscle mass compared to the control group with no effects. Unfortunately, these results cannot be generalized to patients having cancer related skeletal muscle wasting as breast cancer is not typically associated with this syndrome. The other two studies observed the effects of resistance training in patients with androgen deprivation therapy due to prostate cancer. In both studies, resistance training prevented loss of muscle mass and strength seen in patients without exercise training [130, 131]. Nevertheless, because of the small amount of clinical trials, it is difficult to make general conclusions about the effect of resistance training in patients with cachexia due to cancer or other catabolic states.
With respect to exercise training in the elderly, endurance training has a broad application, improving body composition and insulin sensitivity, but with minor effects for strength and muscle mass. In a recently performed randomised study, the different responses to eccentric and concentric training in older men and women were anlyzed [132]. The authors concluded that resistance and endurance training are beneficial for the elderly with regard to muscle function and structural improvements.
7.2 Molecular biological effects of exercise training
But what are the effects of exercise training within the muscle? The anabolic effects of exercise training may be mediated by cytokines, namely, IL-6. This proinflammatory cytokine is released by muscle contraction in healthy individuals as well as diseased patients for example with prostate cancer or muscle wasted COPD [133, 134]. It exerts anti-inflammatory effects by inhibiting the production of TNF-α and IL-1 in vitro, and is reducing the amount of circulating TNF-α [135, 136]. Additionally, exercise training seems to have effects on the antioxidative capacity as it increases the activity of radical scavenger enzymes [137].
Another mechanism of exercise training seems to be mediated via PGC-1α. Increased muscular activity induces PGC-1α production, which in turn protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription [12]. Recently, it has been shown that progressive resistance training may increase muscle protein synthesis by increasing the phosphorylation of mTOR and p70S6k [138, 139]. One acute bout of resistance exercise resulted in significant phosphorylation of mTOR in the plantaris and tibialis anterior muscle of rats. In a healthy young man, an acute bout of low-intensity exercise enhanced mTOR signalling in the vastus lateralis muscle. This was associated with an increased protein synthesis within the skeletal muscle. It has also been shown that both resistance and endurance training blunted the increase in disease-induced muscle proteolysis and improved phosphorylation of Akt and the forkhead transcription factor FoxO1 in a mice model of CKD [140]. Resistance training, but not endurance exercise, corrected protein synthesis and levels of mediators of protein synthesis such as phosphorylated mTOR and p70S6K in the muscles of mice with CKD. Additionally, in these mice, muscle progenitor cell number and activity, as measured by the amounts of MyoD, myogenin and eMyHC mRNAs, were increased.
Another factor influenced by exercise training is myostatin. In an animal model of CHF, we documented a reduction of myostatin concentration after 4 weeks of endurance training in skeletal and heart muscle compared to sham operated animals who underwent exercise [141]. A recently published study reported that exercise training in cachectic and non-cachectic patients with COPD led to an increased IGF-mRNA and protein expression and an increased MyoD concentration. Interestingly, myostatin was down-regulated at mRNA and protein level only in non-cachectic patients [142]. Surprisingly, no effect on TNF-α expression could be detected, but the activation of the transcription factor NFκB was decreased in both groups. MafBx and MuRF-1 expression was increased in cachectic COPD, but it was decreased in non-cachectic patients.
To summarize these data, exercise training is acting in a variety of modes (Fig. 2b), of which the majority is possibly still unknown. Looking at all the collected data, it is impossible to comment on the importance of each affected pathway. They all seem to interact with each other, also depending finally on the genotype of the individual patient. As exercise training has its positive effects, we need to underline this with hard data to put it on a scientific basis and be able to offer it to all affected patients.
References
Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–89.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63.
Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol. 1977;43:1001–6.
Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653–63.
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9.
Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–43.
Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. FOXO transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412.
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–71.
Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 2010;41:110–3.
Lorite MJ, Smith HJ, Arnold JA, Morris A, Thompson MG, Tisdale MJ. Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Br J Cancer. 2001;85:297–302.
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. PGC-1α¦ protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–5.
Glass DJ. Signaling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90.
Guttridge DC. Signaling pathways weigh in on decisions to make or to break skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:443–50.
Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–905.
Bardag-Gorce F, Farout L, Veyrat-Durebex C, Briand Y, Briand M. Changes in 20 S proteasome activity during ageing of the LOU rat. Mol Biol Rep. 1999;26:89–93.
Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margarites I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev. 2006;127:794–801.
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–12.
DeRuisseau KC, AN KAVAZIS, Powers SK. Selective downregulation of ubiquitin conjugation cascade mRNA occurs in the senescent rat soleus muscle. Exp Gerontol. 2005;40:526–31.
Edström E, Altun M, Hägglund M, Ulfhake B. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. J Gerontol A Biol Sci Med Sci. 2006;61:663–74.
Du XL, Sui GZ, Stockklauser-Farber K, Weiss J, Zink S, Schwippert B, et al. Introduction of apoptosis by high proinsulin and glucose in cultured human umbilical vein endothelial cells is mediated by reactive oxygen species. Diabetologia. 1998;41:249–56.
Bossola M, Pacelli F, Costelli P, Tortorelli A, Rosa F, Doglietto G. Proteasome activities in the rectus abdominis muscle of young and older individuals. Biogerontology. 2008;9:261–8.
Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801.
Wendt A, Thompson VF, Goll DE. Interaction of calpastatin with calpain: a review. Biol Chem. 2004;385:465–72.
Dargelos E, Brule C, Combaret L, Hadj-Sassi A, Dulong S, Poussard S, et al. Involvement of the calcium-dependent proteolytic system in skeletal muscle aging. Exp Gerontol. 2007;42:1088–98.
Pattison JS, Folk LC, Madsen RW, Childs TE, Booth FW. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol Genomics. 2003;15:34–43.
O’Connell K, Gannon J, Doran P, Ohlendieck K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int J Mol Med. 2007;20:145–53.
Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200.
Dennis RA, Przybyla B, Gurley C, Kortebein PM, Simpson P, Sullivan DH, et al. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics. 2008;32:393–400.
Welle S, Bhatt K, Shah B, Thornton CA. Insulin-like growth factor-1 and myostatin mRNA expression in muscle: comparison between 62–77 and 21–31 year old men. Exp Gerontol. 2002;37:833–9.
Williams A, Sun X, Fischer JE, Hasselgren PO. The expression of genes in the ubiquitin–proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery. 1999;126:744–9.
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos VE, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51.
Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–8.
Liu CM, Yang Z, Liu CW, Wang R, Tien P, Dale R, et al. Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Ther. 2007;14:945–52.
Jagoe RT, Redfern CP, Roberts RG, Gibson GJ, Goodship TH. Skeletal muscle mRNA for cathepsin B but not components of the ubiquitin–proteasome pathway are increased in patients with lung cancer referred for thoracotomy. Clin Sci. 2002;102:353–61.
Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, et al. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2006;291:R674–83.
Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivray C, et al. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left ventricular dysfunction. Circ Res. 2005;97:418–26.
Eley HL, Russell ST, Tisdale MJ. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem J. 2007;407:113–20.
Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–016.
Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–65.
Li YP, Schwartz RJ, Wadell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NFkB activation in response to tumor necrosis factor a. FASEB J. 1998;12:871–80.
Laviano A, Meguid MM, Preziosa I, Fanelli FR. Oxidative stress and wasting in cancer. Curr Opin Clin Nutr Metab Care. 2007;10:449–56.
Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285:C806–12.
Gomes-Marcondes MC, Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. 2002;180:69–74.
Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009;84:468–81.
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300.
Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63.
Feng J, Xie H, Meany DL, Thompson LV, Arriaga EA, Griffin TJ. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. J Gerontol A Biol Sci Med Sci. 2010;63:1137–52.
Howard C, Ferrucci L, Sun K, Fried LP, Walston J, Varadhan R, et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol. 2007;103:17–20.
Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311.
Barreiro E, Coronell C, Lavina B, Ramirez-Sarmiento A, Orozco-Levi M, Gea J. Aging, sex differences, and oxidative stress in human respiratory and limb muscles. Free Radic Biol Med. 2006;41:797–809.
Fugere NA, Ferrington DA, Tompson LV. Protein nitration with aging in the rat semimembranosus and soleus muscles. J Gerontol A Biol Sci Med Sci. 2006;61:806–12.
Thompson LV, Durand D, Fugere NA, Ferrington DA. Myosin and actin expression and oxidation in aging muscle. J Appl Physiol. 2006;101:1581–7.
Kanski J, Hong SJ, Schoeneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J Biol Chem. 2005;280:24261–6.
Donoghue P, Staunton L, Mullen E, Manning G, Ohlendieck K. DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J Proteomics. 2010;73:1441–53.
Barreiro E, de la Puente B, Busquets S, Lοpez-Soriano FJ, Gea J, Argiles JM. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005;579:1646–52.
Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso M, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–73.
Levine B, Kalman J, Mayer L, Fillit HM, Packer MP. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41.
Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin Jr AS. NF-kappa B-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–6.
Li YP, Reid MB. NF-kB mediates the protein loss induced by TNF-α in differentiated skeletal muscle. Am J Physiol. 2000;279:R1165–70.
Adams V, Linke A, Wisloff U, Doring C, Erbs S, Krankel N, et al. Myocardial expression of Murf-1 and MAFbx after induction of chronic heart failure: effect on myocardial contractility. Cardiovasc Res. 2007;73:120–9.
Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–70.
Adams V, Mangner N, Gasch A, Krohne C, Gielen S, Hirner S, et al. Induction of MuRF1 Is essential for TNF-[alpha]-induced loss of muscle function in mice. J Mol Biol. 2008;384:48–59.
Tisdale MJ. BIOMEDICINE: protein loss in cancer cachexia. Science. 2000;289:2293–4.
Schaap LA, Pluijm SMF, Deeg DJH, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64A:1183–9.
Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–92.
Balage M, Averous J, Remond D, Bos C, Pujos-Guillot E, Papet I, et al. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2010;21:325–31.
Argiles JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999;19:223–48.
Llovera M, Garcia-Martinez C, Lopez-Soriano J, Carbo N, Agell N, Lopez-Soriano FJ, et al. Role of TNF receptor 1 in protein turnover during cancer cachexia using gene knockout mice. Mol Cell Endocrinol. 1998;142:183–9.
Adams V, Nehrhoff B, Späte U, Linke A, Schulze PC, Baur A, et al. Induction of iNOS-expression in skeletal muscle by IL-1β and NFκβ activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54:95–104.
Ungureanu-Longrois D, Balligand JL, Kelly RA, Smith TW. Myocardial contractility dysfunction in the systemic inflammatory response syndrome: role of a cytokine-inducible nitric oxide synthase in cardiac myocytes. J Mol Cell Cardiol. 1995;27:155–67.
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5.
DEcary S, Mouly V, Hamida CB, Sautet A, Barbet JP, Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–38.
Olson EN, Klein WH. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;1:1–8.
Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–83.
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem. 2005;280:2847–56.
McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, et al. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118:3531–41.
McFarlane C, Hennebry A, Thomas M, Plummer E, Ling N, Sharma M, et al. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res. 2008;314:317–29.
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–86.
Zaccagnini G, Martelli F, Magenta A, Cencioni C, Fasanaro P, Nicoletti C, et al. p66ShcA and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hind limb ischemia. J Biol Chem. 2007;282:31453–9.
Adams V, Gielen S, Hambrecht R, Schuler G. Apoptosis in skeletal muscle. Front Biosci. 2001;6:d1–d11.
Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23.
Argiles JM, Lopez-Soriano FJ, Busquets S. Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int J Biochem Cell Biol. 2008;40:1674–8.
Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res. 1977;185:399–408.
Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve. 1983;6:574–80.
Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;20:120–7.
Brooks NE, Schuenke MD, Hikida RS. No change in skeletal mucle satellite cells in young and aging rat soleus muscle. J Physiol Sci. 2009;59:465–71.
Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, et al. Skeletal muscle satellite cell populations in healthy young and older men and women. Anat Rec. 2000;260:351–8.
Leiter JRS, Anderson JE. Satellite cells are increasingly refractory to activation by nitric oxide and stretch in aged mouse-muscle cultures. Int J Biochem Cell Biol. 2010;42:132–6.
Fulle S, Di Donna S, Puglielli C, Pietrangelo T, Beccafico S, Bellomo R, et al. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40:189–97.
Haddad F, Adams GR. Aging-sensitive cellular and molecular mechnisms associated with skeletal muscle hypertroph. J Appl Physiol. 2006;100:1188–203.
Kawada S, Tachi C, Ishii N. Content and localization of myostatin in mouse skeletal muscles during aging, mechanical unloading and reloading. J Muscle Res Cell Motil. 2001;22:627–33.
Leger B, Derave W, DeBock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11:163–75.
Baumann AP, Ibebunjo C, Grasser WA, Paralker VM. Myostatin expression in age and denervation-induced skeletal muscle atrophy. J Musculoskelet Neuronal Interact. 2003;3:8–16.
Chabi B, Ljubicic V, Menzies KJ, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12.
Rice KM, Blough ER. Sarcopenia-related apoptosis is regulated differently in fast- and slow-twitch muscles of the aging F344/N × BN rat model. Mech Ageing Dev. 2006;127:670–9.
Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45:138–48.
Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–9.
Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci USA. 2004;101:1206–10.
Benny Klimek ME, Aydogdu T, link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391:1548–54.
Bonetto A, Penna F, Minero VG, Reffo P, Bonelli G, Baccino FM, et al. Deacetylase inhibitors modulate myostatin/follistatin axis without improving cachexia in tumor-bearing mice. Curr Cancer Drug Targets. 2009;9:608–19.
van Royen M, Carbo N, Busquets S, Alvarez B, Quinn LS, Lopez-Soriano FJ, et al. DNA fragmentation occurs in skeletal muscle during tumor growth: a link with cancer cachexia? Biochem Biophys Res Commun. 2000;270:533–7.
Busquets S, Deans C, Figueras M, Moore-Carrasco R, Lopez-Soriano FJ, Fearon KCH, et al. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr. 2007;26:614–8.
Belizario JE, Lorite MJ, Tisdale MJ. Cleavage of caspases-1, -3, -6, -8 and -9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br J Cancer. 2001;84:1135–40.
Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37.
Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–85.
Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–64.
Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol. 2001;56:M266–72.
Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53.
Sih R, Morley JE, Kaiser FE, Perry III HM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–7.
Bakhshi V, Elliott M, Gentili A, Godschalk M, Mulligan T. Testosterone improves rehabilitation outcomes in ill older men. J Am Geriatr Soc. 2000;48:550–3.
Tenover JL. Testosterone replacement therapy in older adult men. Int J Androl. 1999;22:300–6.
Clague JE, Wu FC, Horan MA. Difficulties in measuring the effects of testosterone replacement therapy on muscle function in older men. Int J Androl. 1999;22:261–5.
Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, Hanks JB, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87:5649–57.
Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab. 1995;269:E820–6.
Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–7.
Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982;42:2495–8.
Zitzmann M, Nieschlag E. Hormone substitution in male hypogonadism. Mol Cell Endocrinol. 2000;161:73–88.
Lobo RA. Androgens in postmenopausal women: production, possible role, and replacement options. Obstet Gynecol Surv. 2001;56:361–76.
Hein L, Barsh GS, Bratt RE, Dzau VJ, Koblika BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2-receptor gene in mice. Nature. 1995;377:744–7.
Garcia JM, Li H, Mann D, Epner D, Hayes TG, Marcelli M, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–91.
Jones TE, Stephenson KW, King JG, Knight KR, Marshall TL, Scott WB. Sarcopenia—mechanisms and treatments. J Geriatr Phys Ther. 2009;32:39–45.
Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24:991–1000.
Dimeo FC, Stieglitz RD, Novello-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–7.
Nieman DC, Cook VD, Henson DA, Suttles J, Rejeski WJ, Ribisl PM, et al. Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. Int J Sports Med. 1995;16:334–7.
Deuster PA, Morrison SD, Ahrens RA. Endurance exercise modifies cachexia and tumor growth in rats. Med Sci Sports Exerc. 1985;17:385–92.
Daneryd P, Hafstroem L, Svanberg E, Karlberg I. Insulin sensitivity, hormonal levels and skeletal muscle protein metabolism in tumour-bearing exercising rats. Eur J Cancer. 1995;31A:97–103.
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–404.
Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomark Prev. 2005;14:1672–80.
Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–9.
Galvao DA, Nosaka K, Taaffe DR, Spry N, Kristjanson LJ, McGuigan MR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38:2045–52.
Mueller M, Breil FA, Vogt M, Steiner R, Lippuner K, Popp A, et al. Different response to eccentric and concentric training in older men and women. Eur J Appl Physiol. 2009;107:145–52.
Van Helvoort HA, Heijdra YF, Thijs HM, Vina J, Wanten GL, Dekhuijzen PN. Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc. 2006;38:1543–52.
Galvao DA, Nosaka K, Taaffe DR, Peake J, Spry N, Suzuki K, et al. Endocrine and immune response to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis. 2008;11:160–5.
Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL- 6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–7.
Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha; production in humans. FASEB J. 2003;17:884–6.
Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, et al. Antioxidative effects of exercise training in patients with chronic heart failure. Increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–70.
Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–10.
Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–7.
Wang XH, Du J, Klein JD, Bailey JL, Mitch WE. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 2009;76:751–9.
Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, et al. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11:342–8.
Vogiatzis I, Simoes DCM, Stratakos G, Kourepini E, Terzis G, Manta P, Athanasopoulos D, Roussos C, Wagner PD, Zakynthinos S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–10.
von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010. doi:10.1007/s13539-010-0003-5
Acknowledgments
All authors of this manuscript comply with the guidelines of ethical authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [143].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lenk, K., Schuler, G. & Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 1, 9–21 (2010). https://doi.org/10.1007/s13539-010-0007-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-010-0007-1