Abstract
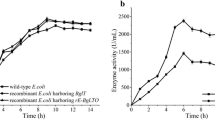
A new fusion gene (Bgl-licMB), encoding β-1,3-1,4-glucanase both from Bacillus amyloliquefaciens (Bgl) and Clostridium thermocellum (licMB), was constructed via end-to-end fusion and expressed in Escherichia coli to improve hydrolytic activity and thermostability of β-1,3-1,4-glucanase. The results of enzymatic properties showed that the catalytic efficiency (Kcat/Km) of the fusion enzyme for oat β-glucan was 2.7 and 20-fold higher than that of the parental Bgl and licMB, respectively, and that the fusion enzyme can retain more than 50% of activity following incubation at 80°C for 30 min, whereas the residual activities of Bgl and licMB were both less than 30%. These properties make this particular β-1,3-1,4-glucanase a good candidate for application in brewing and animal-feed industries.




Similar content being viewed by others
References
Anderson MA, Stone BA (1975) A new substrate for investigating the specificity of β-glucan hydrolases. FEBS Lett 52:202–207
Arai R, Ueda H, Kitayama A, Kamiya N, Nagamune T (2001) Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng 14:529–532
Bedford MR, Patience JF, Classen HL, Inborr J (1992) The effect of dietary enzyme supplementation of rye and barley based diets on digestion and subsequent performance in weanling pigs. Can J Anim Sci 72:97–105
Chen HZ, Li XL, Ljungdahl LG (1997) Sequencing of a 1,3-1,4-β-d-glucanase (lichenase) from the Anaerobic Fungus Orpinomyces Strain PC-2: properties of the enzyme expressed in Escherichia coli and evidence that the gene has a Bacterial origin. J Bacteriol 179:6028–6034
Chen JL, Tsai LC, Wen TN, Tang JB, Yuan HS, Shyur LF (2001) Directed mutagenesis of specific active site residues on Fibrobacter succinogenes 1,3-1,4-β-glucanase significantly affects catalysis and enzyme structural stability. J Biol Chem 276:17895–17901
Han ZL, Han SY, Zheng SP, Lin Y (2009) Enhancing thermostability of a Rhizomucor miehei lipase by engineering a disulfide bond and displaying on the yeast cell surface. Appl Microbiol Biotechnol 85:117–126
Hong MR, Kim YS, Joo AR, Lee JK, Kim YS, Oh DK (2009) Purification and characterization of a thermostable β-1,3-1,4-glucanase from Laetiporus sulphureus var. miniatus. J Microbiol Biotechnol 19:818–822
Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68
Kim SA, Cheng KJ, Liu JH (2002) A variant of Orpinomyces joyonii 1,3-1,4-β-glucanase with increased thermal stability obtained by random mutagenesis and screening. Biosci Biotechnol Biochem 66:171–174
Lee DS, Chang HG (1995) Cloning and expression of a β-1,3-glucanase gene from Bacillus circulans KCTC 3004 in Escherichia coli. Biotechnol Lett 17:355–360
Lee HL, Chang CK, Teng KH, Liang PH (2011) Construction and characterization of different fusion proteins between cellulases and β-glucosidase to improve glucose production and thermostability. Bioresource Technol 102:3973–3976
Li H, Chen J, Li AN, Li DC (2007) Purification and partial characterization of β-1,3-glucanase from Chaetomium thermophilum. World J Microbiol Biotechnol 23:1297–1303
Liu M, Wang J, Liu J, Yao JM, Yu ZL (2006) Expression of Bacillus subtilis JA18 endo-β-1,4-glucanase gene in Escherichia coli and characterization of the recombinant enzyme. Ann Microbiol 56:41–45
Lu P, Feng MG, Li WF, Hu CX (2006) Construction and characterization of a bifunctional fusion enzyme of Bacillus-sourced β-glucanase and xylanase expressed in Escherichia coli. FEMS Microbiol Lett 261:224–230
Luo HY, Yang J, Yang PL, Li J, Huang HQ, Shi PJ, Bai YJ, Wang YR, Fan YL, Yao B (2010) Gene cloning and expression of a new acidic family 7 endo-β-1,3-1,4-glucanase from the acidophilic fungus Bispora sp. MEY-1. Appl Microbiol Biotechnol 85:1015–1023
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungi: their physiology and enzymes. Microbiol Mol Biol Rev 64:461–488
Murray PG, Grassick A, Laffey CD, Cuffe MM, Higgins T, Savage AV, Planas A, Tuohy MG (2001) Isolation and characterization of a thermostable endo-β-glucanase active on 1,3-1,4-β-D-glucans from the aerobic fungus Talaromyces emersonii CBS 814.70. Enzyme Microbiol Technol 29:90–98
Olsen O, Borriss R, Simon O, Thomsen KK (1991) Hybrid Bacillus (1-3,1-4)-β-glucanases: engineering thermostable enzymes by construction of hybrid genes. Mol Gen Genet 225:177–185
Planas A (2000) Bacterial 1,3-1,4-β-glucanases: structure, function and protein engineering. Biochim Biophys Acta 1543:361–382
Qiao JY, Dong B, Li YH, Zhang B, Cao YH (2009) Cloning of a β-1,3-1,4-glucanase gene from Bacillus subtilis MA139 and its functional expression in Escherichia coli. Appl Biochem Biotechnol 152:334–342
Schimming S, Schwarz WH, Staudenbauer WL (1991) Properties of a thermoactive beta-1, 3-1, 4-glucanase (Lichenase) from Clostridium thermocellum expressed in Escherichia coli. Biochem Biophys Res Commun 177:447–452
Seo HS, Koo YJ, Lim JY, Song JT, Kim CH, Kim JK, Lee JS, Choi YD (2000) Characterization of a bifunctional enzyme fusion of trehalose-6-phosphate synthetase and trehalose-6-phosphate phosphatase of Escherichia coli. Appl Environ Microbiol 66:2484–2490
Singh J, Batra N, Sobti RC (2004) Purification and characterization of alkaline cellulase produced by a novel isolate Bacillus sphaericus JS1. J Ind Microbiol Biotechnol 31:51–56
Teng D, Wang JH, Fan Y, Yang YL (2006) Cloning of β-1,3-1,4-glucanase gene from Bacillus licheniformis EGW039 (CGMCC 0635) and its expression in Escherichia coli BL21 (DE3). Appl Microbiol Biotechnol 72:705–712
Wen TN, Chen JL, Lee SH, Yang NS, Shyur LF (2005) A truncated Fibrobacter succinogenes 1,3-1,4-β-glucanase with improved enzymatic activity and thermotolerance. Biochemistry 44:9197–9205
Yang SQ, Yan QJ, Jiang ZQ, Fan GS, Wang L (2008) Biochemical characterization of a novel thermostable β-1,3-1,4-glucanase (lichenase) from Paecilomyces thermophila. J Agric Food Chem 56:5345–5351
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 20090964) and the Wu Xi Science and Technology Program (No. CIE00920).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, J., Wang, H., Lv, W. et al. Construction and characterization of a fusion β-1,3-1,4-glucanase to improve hydrolytic activity and thermostability. Biotechnol Lett 33, 2193–2199 (2011). https://doi.org/10.1007/s10529-011-0676-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0676-7