Abstract
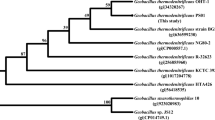
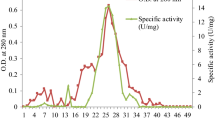
An esterase, Sso2518, from Sulfolobus solfataricus P2 was over-expressed in E. coli. and characterized after purification. The maximum activity was at pH 7.5 and 50°C. The half life of Sso2518 was about 30 min at 85°C and the enzyme was activated by Cu2+. The catalytic triad of Sso2518 was comprised of residues Ser151, Asp176, and His328. Sso2518 showed the highest activity with p-nitrophenyl caproate (C6) and could also hydrolyze olive oil. Under native conditions, Sso2518 consists of 125 kDa homotrimers.


Similar content being viewed by others
References
Abramic M, Lescic I, Korica T (1999) Purification and properties of extracellular lipase from Streptomyces rimosus. Enzyme Microb Technol 25:522–529
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bryant P, Kriek M, Wood RJ, Roach PL (2006) The activity of a thermostable lipoyl synthase from Sulfolobus solfataricus with a synthetic octanoyl substrate. Anal Biochem 351:44–49
Cacciapuoti G, Porcelli M, De Rosa M, Gambacorta A, Bertoldo C, Zappia V (1991) S-adenosylmethionine decarboxylase from the thermophilic archaebacterium Sulfolobus solfataricus. Purification, molecular properties and studies on the covalently bound pyruvate. Eur J Biochem 199:395–400
Ding Y, Cai Y, Zhang G, Xu W (2004) The influence of dipeptide composition on protein thermostability. FEBS Lett 569:284–288
Fisher CL, Pei GK (1997) Modification of a PCR-based site-directed mutagenesis method. Biotechniques 23:570–574
Han D, Krauss G (2009) Characterization of the endonuclease SSO2001 from Sulfolobus solfataricus P2. FEBS Lett 583:771–776
Jaeger KE, Ransac S, Dijkstra BW, Colson C, van Heuvel M, Misset O (1994) Bacterial lipases. FEMS Microbiol 15:29–63
Kim S, Lee SB (2004) Thermostable esterase from a thermoacidophilic archaeon: purification and characterization for enzymatic resolution of a chiral compound. Biosci Biotechnol Biochem 68:2289–2298
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Merone L, Mandrich L, Rossi M, Manco G (2005) A thermostable phosphotriesterase from the archaeon Sulfolobus solfataricus: cloning, overexpression and properties. Extremophiles 9:297–305
Milo RE, Duffner FM, Müller R (1999) Catechol 2, 3-dioxygenase from the thermophilic, phenol-degrading Bacillus thermoleovorans strain A2 has unexpected low thermal stability. Extremophiles 3:185–190
Park Y, Choi SY, Lee HB (2006) A carboxylesterase from the thermoacidophilic archaeon Sulfolobus solfataricus P1; purification, characterization, and expression. Biochim Biophys Acta 1760:820–828
She Q, Singh RK, Confalonieri F et al (2001) The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc Natl Acad Sci 98:7835–7840
Suzuki Y, Miyamoto K, Ohta H (2004) A novel thermostable esterase from the thermoacidophilic archaeon Sulfolobus tokodaii strain 7. FEMS Microbiol Lett 236:97–102
Talker-Huiber D, Jose J, Glieder A, Pressnig M, Stubenrauch G, Schwab H (2003) Esterase EstE from Xanthomonas vesicatoria (Xv_EstE) is an outer membrane protein capable of hydrolyzing long-chain polar esters. Appl Microbiol Biotechnol 61:479–487
Wang J, Cao Y, Zheng G (2009) Mutation in the RGD motif decreases the esterase activity of Xcc_est. Biotechnol Lett 31:1445–1449
Acknowledgement
This work was supported by Youth Fund of State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences and Open fund of State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiayi Wang shared the first author.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10529-010-0299-4
Rights and permissions
About this article
Cite this article
Wang, J., Wang, J., Gong, X. et al. A novel esterase Sso2518 from Sulfolobus solfataricus with a much lower temperature optimum than the growth temperature. Biotechnol Lett 32, 1103–1108 (2010). https://doi.org/10.1007/s10529-010-0261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0261-5