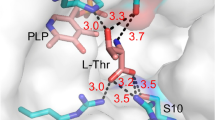
Abstract
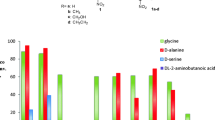
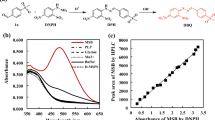
Diastereoselectivity-enhanced mutants of l-threonine aldolase (l-TA) for l-threo-3,4-dihydroxyphenylserine (l-threo-DOPS) synthesis were isolated by error-prone PCR followed by a high-throughput screening. The most improved mutant was achieved from the mutant T3-3mm2, showing a 4-fold increase over the wild-type l-TA. When aldol condensation activity was examined using whole cells of T3-3mm2, its de was constantly maintained at 55% during the batch reactions for 80 h, yielding 3.8 mg l-threo-DOPS/ml.




Similar content being viewed by others
References
Baik SH, Yoshioka H, Yukawa H, Harayama S (2007) Synthesis of l-threo-3, 4-dihydroxyphenylserine (l-threo-DOPS) with thermostabilized low-specific l-threonine aldolase from Streptomyces coelicolor A3(2). J Microbiol Biotechnol 17:1325–1329
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:48–254
Fessner WD, Helaine V (2001) Biocatalytic synthesis of hydroxylated natural products using aldolases and related enzymes. Curr Opin Biotechnol 12:574–586
Herbert RB, Wilkinson B, Ellames GJ, Kunec EK (1993) Streospecific lysis of a range of β-hydroxy-α-amino acids catalyzed by a novel aldolase from S. amakusaensis. J Chem Soc Chem Commun 205–206
Lee SJ, Kang HY, Lee YH (2003) High-throughput screening methods for selecting l-threonine aldolases with improved activity. J Mol Catal B 26:265–272
Liu JQ, Dairi T, Itoh N, Kataoka M, Shimizu S, Yamada H (1998a) Gene cloning, biochemical characterization and physiological role of a thermostable low-specificity l-threonine aldolase from Escherichia coli. Eur J Biochem 255:220–226
Liu JQ, Ito S, Dairi T, Itoh N, Shimizu S, Yamada H (1998b) Low-specific l-threonine aldolase of Pseudomonas sp. NCIMB 10558: purification, characterization and its application to β-hydroxy-α-amino acid synthesis. Appl Microbiol Biotechnol 49:702–708
Liu JQ, Dairi T, Itoh N, Kataoka M, Shimizu S, Yamada H (2000a) Diversity of microbial threonine aldolases and their application. J Mol Catal B 10:107–115
Liu JQ, Odani M, Yasuoka T, Dairi T, Itoh N, Kataoka M, Shimizu S, Yamada H (2000b) Gene cloning and overexpression of low-specific d-threonine aldolase from Alcaligenes xylosoxidans and its application for production of a key intermediate for parkinsonism drug. Appl Microbiol Biotechnol 54:44–51
Ohashi N, Nagata S, Ishizumi K, Maeshima K (1984) Process for producing l-threo-3(3,4-dihydroxyphenyl)serine. European Patent 0084928
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts MG, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409:258–268
Schoemaker HE, Mink D, Wubbolts MG (2003) Dispelling the myths–biocatalysis in industrial synthesis. Science 14:1694–1697
Straathof AJ, Panke S, Schmid A (2002) The production of fine chemicals by biotransformation. Curr Opin Biotechnol 13:548–556
Vassilev VP, Uchiyama T, Kajimoto T, Wong CH (1995) l-Threonine aldolase in organic synthesis: preparation of novel β-hydroxy-α-amino acids. Tetrahedron Lett 36:4081–4084
Wong CH, Whitesides GM (1994) Enzymes in synthetic organic chemistry. Pergamon, Oxford
Acknowledgments
We thank Ms. Tomiki Aki for her technical assistance. This work was supported by partly Chonbuk National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gwon, HJ., Baik, SH. Diastereoselective synthesis of l-threo-3,4-dihydroxyphenylserine by low-specific l-threonine aldolase mutants. Biotechnol Lett 32, 143–149 (2010). https://doi.org/10.1007/s10529-009-0125-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0125-z