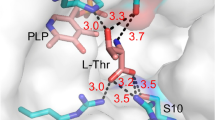
Abstract
The direct biochemical synthesis of tertiary α-amino acids with a wide range of diversity was recently reported using natural threonine aldolases LTA from Aeromonas jandei and DTA from Pseudomonas sp. Here, we describe the identification of five novel threonine aldolases which accept alanine and serine as amino acid donors. The enzymes were found by sequence database analysis using known aldolases as template. All enzymes were overexpressed in Escherichia coli and purified, and their biochemical properties were characterized. The new enantiocomplementary l- and d-threonine aldolases catalyze the asymmetric synthesis of β-hydroxy α-methyl- and α-hydroxymethyl-α-amino acids with good conversion and perfect enantioselectivity at α-carbon of the products (e.e. >99 %). The structural basis for the broad donor specificity of these threonine aldolases is analyzed based on crystal structure alignments and amino acid sequences comparison.




Similar content being viewed by others
References
Avenoza A, Busto JH, Corzana F, Peregrina JM, Sucunza D, Zurbano MM (2004) α-Methylserinals as an access to α-methyl-β-hydroxy amino acids: application in the synthesis of all stereoisomers of α-methylthreonine. Tetrahedron Asymmetry 15:719–724
Baker P, Seah SYK (2012) Rational approaches for engineering novel functionalities in carbon-carbon bond forming enzymes. Comput Struct Biotechnol J 2:1–10
Bornscheuer UT, Kazlauskas RJ (2004) Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew Chem Int Ed 43:6032–6040
Clapes P, Garrabou X (2011) Current trends in asymmetric synthesis with aldolases. Adv Synth Catal 353:2263–2283
Davids T, Schmidt M, Böttcher D, Bornscheuer UT (2013) Strategies for the discovery and engineering of enzymes for biocatalysis. Curr Opin Chem Biol 17:215–220
di Salvo ML, Soumya G, Remesh M, Vivoli M, Ghatge MS, Paiardini A, D’Aguanno S, Safo MK, Contestabile R (2014) On the catalytic mechanism and stereospecificity of Escherichia coli L-threonine aldolase. FEBS J 281:129–145
Dietz F, Gröger H (2009) Asymmetric synthesis of all stereoisomers of α-methylthreonine using an organocatalytic steglich rearrangement reaction as a key step. Synlett 24:4208–4218
Dückers N, Baer K, Simon S, Gröger H, Hummel W (2010) Threonine aldolases -screening, properties and applications in the synthesis of non-proteinogenic β-hydroxy-α-amino acids. Appl Microbiol Biotechnol 88:409–424
Fesko K, Gruber M (2013) Biocatalytic methods for C-C bond formation. ChemCatChem 5:1248
Fesko K, Reisinger C, Steinreiber J, Weber H, Schürmann M, Griengl H (2008) Four types of threonine aldolases: similarities and differences in kinetics/thermodynamics. J Mol Catal B 52–53:19–26
Fesko K, Uhl M, Steinreiber J, Gruber K, Griengl H (2010) Biocatalytic access to α, α-dialkyl-α-amino acids by a mechanism-based approach. Angew Chem Int Ed 49:121–124
Fessner WD (2011) Aldol reactions. In: Drauz K, Gröger H, May O (eds) Enzyme catalysis in organic synthesis, 3rd edn. Wiley-VCH, Weinheim, pp 857–917
Gatti-Lafranconi P, Hollfelder F (2013) Flexibility and reactivity in promiscuous enzymes. ChemBioChem 14:285–292
Grandel R, Kazmaier U (1998) Diastereoselective synthesis of β-substituted α-methylserines via alanine ester enolates. Eur J Org Chem 2:409–417
Hernandez K, Zelen I, Petrillo G, Uson I, Wandtke CM, Bujons J, Joglar J, Parella T, Clapes P (2015) Engineered L-serine hydroxymethyltransferase from Streptococcus thermophilius for the synthesis of α, α-dialkyl-α-amino acids. Angew Chem Int Ed 54:3013–3017
Kataoka M, Wada M, Nishi K, Yamada H, Shimizu S (1997) Purification and characterization of L-allo-threonine aldolase from Aeromonas jandaei DK-39. FEMS Microbiol Lett 151(2):245–248
Kielkopf CL, Burley SK (2002) X-ray structures of threonine aldolase complexes: structural basis of substrate recognition. Biochemistry 41:11711–11720
Müller M (2012) Recent developments in enzymatic asymmetric C-C bond formation. Adv Synth Catal 354:3161–3174
Qin HM, Imai FL, Miyakawa T, Kataoka M, Okai M, Ohtsuka J, Hou F, Nagata K, Shimizu S, Tanokura M (2014) L-allo-Threonine aldolase with an H128Y/S292R mutation from Aeromonas jandaei DK-39 reveals the structural basis of changes in substrate stereoselectivity. Acta Crystallogr Sect D 70:1695–1703
Reisinger C, Kern A, Fesko K, Schwab H (2007) An efficient plasmid vector for expression cloning of large numbers of PCR fragments in Escherichia coli. Appl Microbiol Biotechnol 77:241–244
Steinreiber J, Fesko K, Reisinger C, Schürmann M, Assema F, Wolberg M, Mink D, Griengl H (2007a) Threonine aldolases - an emerging tool for organic synthesis. Tetrahedron 63:918–926
Steinreiber J, Fesko K, Reisinger C, Schürmann M, Assema F, Griengl H (2007b) Synthesis of γ-halogenated and long-chain β-hydroxy-α-amino acids and 2-amino-1,3-diols using threonine aldolases. Tetrahedron 63:8088–8093
Toney MD (2005) Reaction specificity in pyridoxal phosphate enzymes. Arch Biochem Biophys 433:279–287
Vogt H, Bräse S (2007) Recent approaches towards the asymmetric synthesis of α, α-disubstituted α-amino acids. Org Biomol Chem 5:406–430
Wildmann M, Pleiss J, Samland AK (2012) Computational tools for rational protein design of aldolases. Comput Struct Biotechnol J 2:1–11
Windle C, Müller M, Nelson A, Berry A (2014) Engineering aldolases as biocatalysts. Curr Opin Chem Biol 19:25–33
Zhang WH, Otting G, Jackson CJ (2013) Protein engineering with unnatural amino acids. Curr Opin Struct Biol 23:581–587
Acknowledgments
The activity leading to the present results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution for the Innovative Medicine Initiative under Grant Agreement No. 115360 (Chemical manufacturing methods for the 21st century pharmaceutical industries, CHEM21). We would like to thank Dr. Martina Geier for the support with genetic experiments.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fesko, K., Strohmeier, G.A. & Breinbauer, R. Expanding the threonine aldolase toolbox for the asymmetric synthesis of tertiary α-amino acids. Appl Microbiol Biotechnol 99, 9651–9661 (2015). https://doi.org/10.1007/s00253-015-6803-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6803-y