Abstract
l-lactate is a catabolite from the anaerobic metabolism of glucose, which plays a paramount role as a signaling molecule in various steps of the cell survival. Its activity, as a master tuner of many mechanisms underlying the aging process, for example in the skin, is still presumptive, however its crucial position in the complex cross-talk between mitochondria and the process of cell survival, should suggest that l-lactate may be not a simple waste product but a fine regulator of the aging/survival machinery, probably via mito-hormesis. Actually, emerging evidence is highlighting that ROS are crucial in the signaling of skin health, including mechanisms underlying wound repair, renewal and aging. The ROS, including superoxide anion, hydrogen peroxide, and nitric oxide, play both beneficial and detrimental roles depending upon their levels and cellular microenvironment. Physiological ROS levels are essential for cutaneous health and the wound repair process. Aberrant redox signaling activity drives chronic skin disease in elderly. On the contrary, impaired redox modulation, due to enhanced ROS generation and/or reduced levels of antioxidant defense, suppresses wound healing via promoting lymphatic/vascular endothelial cell apoptosis and death. This review tries to elucidate this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: l-lactate as a signaling molecule
l-lactate, is known also as l-lactic acid, i.e., an α-hydroxyl acid and is a ubiquitous molecule coming from the reduction of l-pyruvate into l-lactate by the l-lactate dehydrogenase (LDH, E.C. 1.1.1.27), to produce NAD + from NADH in the Embden–Meyerhof–Parnas’s pathway. l-lactate is commonly considered an apparent useless byproduct of glycolysis (Rogatzki et al. 2015). Actually, since many years, l-lactate has been mistakenly described as simply a toxic remnant of the anaerobic metabolism, despite recent evidence would suggest a crucial role for l-lactate in the cell biology (Ratter et al. 2018; Philp et al. 2005; Brooks 2020; Manosalva et al. 2022).
This l-enantiomer can be formed even under aerobic conditions with a specific signaling function (Brooks 2020; Baltazar et al. 2020; Tauffenberger et al. 2019). As outlined in this overview, l-lactate serves as a master regulator of many complex pathways regulating crucial cell functions. Its ability to exert fundamental signaling functions, sets l-lactate within the major crucial pathways of cell survival (Lee 2021).
However, despite its crucial role in many steps of the metabolic check-point leading to cell survival and differentiation, a relatively scant literature exists about l-lactate as an on/off switcher of major intracellular pathways, involved in aging and survival of skin. A survey on Pubmed/Medline with the MESH term “lactate AND aging AND signaling” retrieved 283 releases, yet only 11 if the term “skin” is added. Despite the wide use of l-lactate in many cosmetic formulations, the role of this molecule in regulating skin function and skin senescence is yet far to be fully accomplished (Huang et al. 2020).
The modulating activity of l-lactate is closely linked with its ability to work as a signaling factor in crucial steps of the cell machinery. Usually, the evolution has selected small, ubiquitous and pleiotropic molecules to act as signaling molecules, for example reactive oxygen species (ROS) (D'Autréaux and Toledano 2007), nitric oxide (NO) (Tuteja et al. 2004), adenosine from ATP (Eltzschig 2013), carbon monoxide (Mann 2010) and so forth. In particular, carbon monoxide (CO) is an endogenously derived gas formed from the breakdown of haeme by the enzyme haeme oxygenase 1 (HO-1). Although long considered an insignificant and potentially toxic waste product of haeme catabolism, CO is now recognized as a key signaling molecule that regulates numerous metabolic functions including the many cytoprotective, antioxidant, and anti-inflammatory abilities (Durante et al. 2006; Kim et al. 2006).
l-lactate role in the aging process and the role of mitochondria
In recent years, despite the role of l-lactate has been widely associated, as a biomarker, with the noxious activity of stressors, xenobiotics and endogenous toxicants (Seheult et al. 2017; Schmidt and Karlson-Stiber 2008; Manojlović and Erčulj 2019), evidence was reported about a surprising beneficial activity of this α-hydroxyl acid. For example, l-lactate protects skin fibroblasts from mitochondria aging-related dysfunction, via a process known as “mito-hormesis” (Zelenka et al. 2015).
Mito-hormesis (Barzegari et al. 2022; Bárcena et al. 2018; Bordon 2021) is a modulating process, involving mitochondria and the mitochondria-associated membrane (MAM) system (van Vliet and Agostinis 2018; van Vliet et al. 2014), where a “mild” stress induction can lead to a persistent cellular adaptation to stressors. This adaptation enables the cell to prevent damage, enhance its survival response and activate rejuvenation and/or biogenesis processes, in order to protect the cell from apoptosis and mitochondria from dysfunction.
As a matter of fact, mitochondria may reduce the impact of reactive oxygen species (ROS)-mediated damage by uncoupling the oxidative phosphorylation via the uncoupling protein-1 but also by modulating the level of ROS as signaling molecules by means of the mito-hormesis (Atayik and Çakatay 2022). The role of l-lactate in this process is yet under investigation. Mito-hormesis can be considered as an adaptive stress response via a kind of mito-nuclear signaling, acting in order to enhance cell survival and stress resistance, for example by inducing the release of factors such as fibroblast growth factor 21 (FGF21) and growth and differentiation factor 15 (GDF15), two fundamental stress-triggered mitokines (Klaus and Ost 2020), to cite l-lactate role in the skin.
If l-lactate has a beneficial role on cell survival via a mito-hormetic mechanism, then it should lead to a fundamental modulation of the ROS signaling. In this context, l-lactate regulation of the stress response, and hence of cell survival and differentiation, appears particularly intriguing also to elucidate the aging process, if l-lactate is at the same time a waste product and a major signaling molecule.
More generally, it is tempting to speculate that one of the major roles of highly ubiquitous and largely pleiotropic molecules, such as CO and l-lactate, usually coming from the complex metabolic network of signaling and enzymatic pathways, is to be widely used as fine regulators. Therefore, as occurring with ROS, ATP, adenosine, calcium ions and so on, l-lactate too, should be re-interpreted as a signaling molecule with fundamental modulatory actions (Coggan et al. 2022; Veloz Castillo et al. 2021).
During the aging process, l-lactate exerts fundamental actions in the brain, as it has been recently considered also a biomarker of brain senescence, where the ratio LDH isoenzymes A/B may be altered, though controversial opinions yet remain (Ross et al. 2010; Datta and Chakrabarti 2018). In a recent mitochondrial theory of aging, dating back to several years, mitochondria dysfunction and damage in the mitochondrial DNA (mtDNA), are collectively included in the causative panoply of factors leading to the aging process of cells and tissues (Harman 1972; Larsson 2010). This might elucidate the possible role of l-lactate in the subsequent impairment of mitochondria activity, leading the cell to switch mainly towards aerobic mode of fermentation, so regulating fundamental pathways in the survival machinery.
Prematurely aged mice with mutated mtDNA, have mitochondria shifting aerobic metabolism to a glycolytic pathway (Ross et al. 2010). The intrinsic meaning of this mechanism is still under investigation, yet it involves l-lactate, and suggests a role for this molecule as a master regulator of mitochondria biology (Ross et al. 2010). As a matter of fact, the role of mitochondria in aging has been widely confirmed in the most recent literature (Sun et al. 2016; Srivastava 2017; Jang et al. 2018). In this perspective, l-lactate may be a leading factor in the adjusting of mitochondria physiology (Van Hall 2000; Levasseur et al. 2006). Interestingly, recent data reported that an intermittent treatment of skin fibroblasts with l-lactate induced a mild inhibition of the mitochondrial aerobic process via the respiratory chain (mito-hormesis), causing production of hydrogen peroxide, phosphorylation of AMPK and activation of the mitochondria biogenesis via PGC-1α, so activating the cellular endowment of survival genes and enzymes and finally acting as a skin protective factor (Ross et al. 2010). Hydrogen peroxide is involved in the intracellular signaling of ROS, acting as a mediator of several physiological processes such as cell differentiation and proliferation, cellular metabolism, survival, and immune response (Di Marzo et al. 2018).
This evidence should suggest that moderate levels of l-lactate, for example during muscular exercise, may have beneficial effects, allowing to consider l-lactate as a signaling molecule in the complex role exerted by mitochondria in the aging process, using sustained, moderate exercise (Musci et al. 2019; Merry and Ristow 2016).
However, the role of l-lactate in muscles might include a much wider complex of signaling factors and pathways, which encourages researchers to define its task in a more systemic landscape than the simplest biochemical machinery associated with mitochondria. Past reports, showing a beneficial action of topical l-lactate on skin biology, might have a possible elucidation by the most recent research in the field (Smith 1996; Tran et al. 2014; Huang et al. 2020).
l-lactate may be closely related to aging as, in animal models, the level of this glycolytic byproduct rapidly increases in the senescent phenotype (Datta and Chakrabarti 2018; Wallace et al. 2020).
Senescent cells exhibit higher glycolytic activity and lactate production than youngest cells, alongside with an enhanced expression of lactate dehydrogenase A as well as increases in tricarboxylic acid cycle activity and mitochondrial respiration. The latter is likely due to the reduced expression of pyruvate dehydrogenase kinases (PDHKs) in senescent cells, which may lead to increased activity of the pyruvate dehydrogenase complex (Stabenow et al. 2022).
At least in murine models, the circadian rhythms of lactate in aged C57BL/6N male mice (19 months) appear slightly phase-advanced respect to younger animals, affecting the metabolic activity of the prefrontal cortex in the brain (7 months). A possible reason can be hypothesized by observing the reduction in GLUT-1 receptors alongside with the aging process (Wallace et al. 2020). If aging modulates the systemic involvement of l-lactate in the individual’s metabolic homeostasis, then l-lactate should have a fundamental role even in the human physiology.
Actually, circadian rhythms of l-lactate were reported also in humans, particularly during physical exercise (Forsyth and Reilly 2004; Reilly and Waterhouse 2009), and furthermore recent evidence suggests that a cross talk between metabolism and circadian rhythms can be described (Reinke and Asher 2019).
At a cellular level, where cells should have their own circadian oscillators, an interplay dynamic between circadian clocks and the mammalian target of rapamycin (mTOR) pathway was reported (Guerrero-Morín and Santillán 2020). This perspective suggests that the role of l-lactate as a master regulator of aging must be much more systemic that expected.
So far, the linkage between l-lactate, aging and redox modulation, is overshadowed by a huge crowd of molecular participants in the highly complex milieu describing the fundamental role of mitochondria in the cell survival. This might explain why scientific literature is particularly scant in gathering reports showing the role of l-lactate, not exclusively as a catabolite, despite some recent reports on the topic of l-lactate as a signaling molecule (Table 1). In this review we attempt to elucidate the role of l-lactate as a signaling and regulatory molecule in the aging process, focusing particularly on the senescence process of the skin.
Insights about l-lactate as a signaling and modulatory molecule
Role of ROS
Skin aging, health and disease are closely intertwined with mitochondria biology (Sreedhar et al. 2020). As the epidermis is a highly self-renewing tissue, the role of mitochondria may be therefore crucial (Zhang et al. 2018; Stout and Birch-Machin 2019). Furthermore, in this context, ROS play also a modulatory role in differentiating numerous cell lineages via downstream pathways such as C/EBP, β-catenin and Notch, even promoting differentiation in murine embryonic stem cells and the induction of both pluripotent stem cells and multipotent stem cells of epithelial origin (Lisowski et al. 2018). In conditional knock out mice, for the expression of the mitochondria transcription factor A (TFAM), some authors reported a high mortality rate caused by the absence of a correct functionality in the epithelial barrier and primary keratinocytes from these laboratory animals were unable to differentiate in vitro (Hamanaka et al. 2013). Actually, TFAM is involved in leading the replication of mitochondrial DNA and those cells lacking TFAM have impaired oxidative phosphorylation and ROS production (Hamanaka et al. 2013). This perspective strongly suggests that ROS are crucial in the signaling of skin health, including mechanisms underlying wound repair, renewal and aging. The outstanding role of ROS, as signaling molecules for skin health and aging, should be therefore reappraised (Ndiaye et al. 2014; Dunnill et al. 2017; Gauron et al. 2013).
ROS are major contributors in skin renewal, stem cell biology and keratinocyte differentiation. Alongside with ROS, it is presumable that l-lactate might play a role in skin aging and skin renewal, not so differentially from the signaling function exerted by ROS.
In this perspective, the relationship between ROS and l-lactate should be better highlighted.
A recent paper by Tauffenberger et al. reported that l-lactate, as well as l-pyruvate, is able to activate a stress response mechanism by eliciting a hormetic response of ROS, so activating the Nrf2/Keap1/ARE system and the unfolded protein response (UPR) (Tauffenberger et al. 2019). The question one might raise is whether l-lactate would act as a mild stressor, such as plant flavonoids, phytochemicals and other xenobiotics, for example, or if this catabolite has a much higher importance, in the cell economy.
Certainly, as l-lactate is the major product of glycolysis, its role cannot be compared to the occasional beneficial activity of a xenobiotic, due to hormetic mechanisms. Interestingly, aryl hydrocarbon receptors (AhRs), which act as transcription factors, modulate the genetic expression of several enzymes generating uridine monophosphate, alongside with LDHA, therefore controlling l-lactate production (Lafita-Navarro et al. 2020). The close relationship of xenobiotic biology via AhRs and l-lactate levels is suggestive for a signaling role of l-lactate in the oxidative stress response.
Impaired redox modulation of signaling pathways is a leading causative factor of cellular senescence.
Role of other factors: autophagy and apoptosis
It is now well established that a subtle balancing of mitochondria activity via signaling molecules such as ROS and the fine regulation of biochemical pathways leading to the control of cell survival, is the main mechanism causing aging. Probably, aging is not merely an end-oriented process but the breakage of minute intracellular equilibria, which then may lead to an irreversible development and pathophysiology (Liguori et al. 2018; Kruk et al. 2019; Kitada and Koya 2021). The role of l-lactate in autophagy, for example, can provide further insights about its ability to modulate important signaling pathways in cell biology. It is well known that LDH-B and LDH-A isoenzymes control autophagy (Brisson et al. 2016; Das et al. 2019).
Autophagy is a fundamental process to maintain longevity (Barbosa et al. 2019) and recent data reported that l-lactate regulates autophagy, via the ERK1/2/m-TOR/p-70S6K pathway (Nikooie et al. 2021). Even this role in autophagy might have a hormetic cause, as the same l-lactate has an inhibitory action on autophagy when cells have lost their stress responsivity, such as in tumors (Matsuo et al. 2019). Again, in this perspective, l-lactate should exert a fundamental regulatory activity even on apoptosis (Go et al. 2021). At least on HepG2 cell lines, the increase in the extracellular lactate-to-pyruvate ratio has the ability to reduce the cytosol NADH/NAD+ redox state and inhibiting stress-induced apoptosis (Go et al. 2021). Moreover, high extracellular l-lactate inhibits the intrinsic apoptotic pathway by reducing the activation of JNK and Bax (Go et al. 2021).
The effect on the apoptotic pathway is of the utmost importance for aging, as reported by past reports regarding skin senescence (Haake et al. 1998; Zhang and Herman 2002; Tower 2015), but it is more presumably autophagy that mainly controls the process of skin aging (Eckhart et al. 2019). In this perspective, it is tempting to speculate if l-lactate might have a role in skin protection or repair, due to its modulatory activity on these mechanisms.
l-lactate in skin aging
First intriguing evidence is that l-lactate is able to stimulate both the expression of CD44 and hyaluronan in H8 27 human dermal fibroblasts (Stern et al. 2002). The authors reported that the Warburg-like effect, leading to an increase in l-lactate, was probably the consequence of a blood and oxygen reduction in a wound repair mechanism. As a matter of fact, the incubation of H8 27 human fibroblasts with l-lactate enhanced the expression of the hyaluronan receptor CD44 and the production of hyaluronic acid (Stern et al. 2002). During wound repair, l-lactate accumulates and l-lactate itself works as an inducer of ROS to promote dermal fibroblasts growth, via the requirement of iron and hydrogen peroxide (Wagner et al. 2004). Moreover, it is well known that aging has a detrimental effect on skin fibroblasts, both reducing their growth and altering the expression of a wide plethora of collagen, metallothionein, interleukin, caspase and sirtuin genes (Lago and Puzzi 2019). Therefore, l-lactate may even exhibit an anti-aging role (Tran et al. 2014). Finally, l-lactate promotes the shift from the mitochondrial oxidative phosphorylation (OXPHOS) to glycolysis via HIF-1α and stabilizes the activity of the same hypoxic factor HIF-1α, probably in order to support the signaling function of ROS in human fibroblasts (Kozlov et al. 2020).
The relationship between ROS, mitochondria and l-lactate is of utmost importance for the cell survival, involving glucose metabolism as a switching control (Liemburg-Apers et al. 2015). A possible vicious cycle ROS-glycolysis may lead to cell death when ROS signaling is impaired (Liemburg-Apers et al. 2015).
ROS signaling is ruled by H2O2, (Forman et al. 2010) and hydrogen peroxide is crucial for a complex network of such biochemical hubs linking cell metabolism with the aging process (Roger et al. 2020). So, cellular aging may be the puzzling resultant of a complex interplay between ROS and l-lactate, via H2O2 scavenging enzymes such as peroxiredoxins (Roger et al. 2020).
The mechanisms of skin aging have been thoroughly reviewed (Jenkins 2002; Kohl et al. 2011; Zhang and Duan 2018). A key mechanism of skin aging is the induction of ROS and metalloproteinases (Kohl et al. 2011). These components are the major alarming signals of tissue and cell damage and a powerful biomarker of critically illness circumstances.
A close relationship between metalloproteinase-9 (MMP9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP1) with l-lactate in plasma of critically ill patients, has been recently reported (Duda et al. 2020). Aging involves a thorough remodeling of tissues making the skin and matrix metalloproteinases are fundamental actors in the complex turnover of the extracellular matrix (ECM) and of cell composition in the connective tissue (Freitas-Rodríguez et al. 2017). It is presumable that l-lactate might exert a major activity in this complex scenario. Furthermore, in an effort to elucidate which kind of functional relationship l-lactate engages with the complex intracellular milieu of factors regulating ROS signaling and aging, where glucose metabolism might be the major key.
Role of glucose
A past paper by Park et al. highlighted the evidence that a sustained hyperglycemic state, such as occurring during type 2 diabetes, induces an impaired skin barrier state, probably because skin homeostasis is delayed (Park et al. 2011). High glucose induces alterations in sirtuins, causing also a rapid aging in endothelial cells via forkhead transcription factors (FOXO) and p300 regulated pathway (Mortuza et al. 2013). Actually, in diabetic animals, endothelia showed signs of senescence, senescence associated β-gal (SA-β-gal) expression, reduction in sirtuins 1–7 and in FOXO1 DNA binding ability (Mortuza et al. 2013). If excess of glucose is a leading factor of senescence induction (Liu et al. 2020a; Zhang et al. 2017; Danby 2010; Yin et al. 2021), particularly for mesenchymal stem cells (Yin et al. 2021), the role of l-lactate in the regulation of a glycemic-induced senescent phenotype should be particularly interesting. In type 2 diabetic patients, with a chronical hyperglycemic state, (Brouwers et al. 2015; Adelsmayr et al. 2012), l-lactate is a leading biomarker, so it is presumable that l-lactate should play a role as a regulator in the glycemic impact of the stress response.
Two different of fundamental routes are to be put in the spotlight to further elucidate the involvement of l-lactate in the aging process of the skin: (a) the role of l-lactate in bioenergetics and mitochondria biology; (b) the role of l-lactate in stem cell commitment in the skin and mesenchymal differentiation. Both functions are intertwined with ROS biology.
l-lactate in skin physiology
l-lactate and skin biology during aging: the immune mircoenvironment
Due to the fundamental role of l-lactate in the mitochondria-ROS signaling, and therefore in the aging process, skin differentiation should be affected by l-lactate turnover and metabolism. Actually, l-lactate participates in the complex milieu of skin cellular components by interacting with the interplay stromal cells-immune cells, for example by switching off the pro-inflammatory immune response and promoting tissue renewal and repair (Selleri et al. 2016; He et al. 2019). Human stromal cells from mesenchymal origin (MSCs) release l-lactate and induce a lactate-mediated reprogramming in dendritic cells, i.e., MSCs produce large amounts of l-lactate and cause the differentiation of monocytes to M2-macrophages, so acting as an immunomodulant molecule (Selleri et al. 2016). The role of M2-macrophages in aging and skin biology is intriguing, because these anti-inflammatory phenotypes are typically skewed from precursors in tissue repair mechanisms, which encompass the involvement of Th2 cytokines mediating an M2 programming of monocyte-to-dendritic cells and moreover of apoptotic events, then contributing in tissue renewal (Kim and Nair 2019). It is possible that l-lactate works as a switcher in the M1/M2 skewing process, to ensure the ability of monocyte to polarize, as this ability is reduced in advanced senescent phenotypes (Mahbub et al. 2012). The existence of an M2-milieu in the innate immunity of skin, promotes cell differentiation and survival. It is well known that MSCs have a multi-lineage differentiating pattern, in order to improve wound healing, but also recent data reported that MSCs are joined to M2-skewed macrophages and by the co-occurrence of a hypoxic microenvironment (Lee et al. 2016).
Role of hypoxia and staminality
Aging in the skin can be caused by the loss of the hypoxia-inducible factor 1 alpha (HIF-1α) (Rezvani et al. 2011). In normal human diploid BJ fibroblasts, l-lactate promotes the role of HIF-1α in shifting mitochondria oxidative phosphorylation to glycolysis (Kozlov et al. 2020). HIF-1α is a leading regulator of glycolysis and is able to promote the expression of several genes involved in glucose uptake and metabolism, such as pyruvate dehydrogenase kinase (PDK, isozymes 1–3) and pyruvate kinase muscle isozyme 2 PKM2 (Prigione et al. 2014). Usually, HIF-1α is rapidly degraded following its genetic translation in normoxic conditions but l-lactate has the ability to stabilize HIF-1α in the cell, prolonging its action and therefore contribution in reducing the impact of the aging process. This ability, as observed in BJ human fibroblasts is promoted by l-lactate via a ROS signaling (Kozlov et al. 2020). Stabilizing HIF-1α in dermal fibroblasts leads to the enhancement of PDK1 and PKM2 proteins, whereas PDK1 and LDHA are particularly increased in hypoxic conditions (Kozlov et al. 2020). The glycolytic shift is not only a switching on/off on aerobic/anaerobic metabolism but relates mitochondria function and l-lactate to cell replication and stem cell biology. In this perspective, it is interesting to observe that c-myc promotes a state of high energy supply, fundamental for stem cell generation, in which further components such as the estrogen related receptor alpha (ERRα) and its major cofactor, peroxisome proliferator-activator receptor gamma coactivator 1-beta (PGC1-β), are also involved (Prieto et al. 2018; Kida et al. 2015). Furthermore, in early somatic cell reprogramming, a fundamental role is exerted by the snail family of transcriptional repressor (SNAIL1), which has a role in the epithelial-to-mesenchymal (EMT) transition (Unternaehrer et al. 2014). l-lactate increase the expression of c-myc and SNAIL in dermal fibroblasts, so showing the crucial role of this catabolite as a signaling molecule in stem cell reprogramming (Kozlov et al. 2020).
The relationship between aging and MSCs has been recently reviewed (Liu et al. 2020a, b, c). In aged and senescent MSCs a down-regulation in the expression of C–C motif chemokine receptor 7 (CCR7), stromal cell-derived factor 1 (SDF-1) and its receptor chemokine receptor type 4 (CXCR4), and also of tumor necrosis factor receptor (TNFR) and IFN-γ receptor (IFNGR), have been observed (Liu et al. 2020a, b, c). Furthermore, an age-related decline in the gene expression of the runt-related transcription factor 2 (Runx2), the core binding factor α1 (CBFA1), and distal-less homeobox 5 (DIx5) as well as osteocalcin and collagen, has been reported, alongside with an increase in pro-adipogenetic components such as peroxisome proliferator-activated receptor-γ (PPAR-γ) and adipocyte fatty acid-binding protein (aP2) (Jiang et al. 2008). A close relationship between Runx2 and HIF-1α occurs to promote angiogenic signals (Kwon et al. 2011). l-lactate, by stabilizing HIF-1α even in normoxic conditions, promote vascular endothelial growth factor (VEGF) production (Song et al. 2018). In this perspective, therefore, l-lactate may contribute in dermal vascularization, which is a major issue in skin aging. An age-related decrease in dermal vascularization, might be due to impairment in VEGF signaling via the delta-like ligand 4 (Dll4) and Jagged-1 (Jag-1) (Gunin et al. 2014).
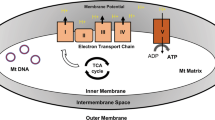
Aging in skin can be considered a degenerating process starting from the increasing difficulty of mitochondria to ensure cells with the ability of promote survival process, stressors scavenging and stem cell/differentiation interplays at a balanced level. Figure 1 shows the possible relationship between l-lactate and mitochondria biology to elucidate the role of l-lactate in the aging process. The central core of this complex task is the ability of l-lactate to join metabolism and bioenergetic with the oscillating ability of mitochondria to regulate the cell fate. ROS are continuously to be adjusted to work as signaling molecules, whereas any excess must be buffered in order to prevent mitochondria stress and the impairment in their biogenesis and turnover. Further research should elucidate the role of l-lactate in this perspective.
A schematic representation of l-lactate activity within the pathogenesis of premature/extrinsic skin aging. In the center of the cartoon is simplified the complex balance on ROS production by mitochondria, in order to assess ROS as signaling molecules. This mechanism allows mitochondria biogenesis, uncoupled events, mitokinesis and mitochondria fission/fusion, via the PGC-1α but l-lactate is also able to use oxidized lipids to trigger the Nrf2/keap1/ARE via mitohormetic mechanism. The activity by l-lactate on HIF-1α allows to bring together two quite opposite but interplaying pathways, i.e. the Nrf2/Keap1/ARE pathway with HO-1 and the MAPK/NF-κB pathway. Aging in the skin is exemplified by events within squares red lined squares, whereas events reverting the aging process and promoting survival and renewal are green lined squares. l-lactate is indicated as “l” within a green circle (if promoting or triggering), pale yellow if regulating, red if inhibiting. DAMP damage associated molecular pattern, HO-1 heme oxygenase-1, NF-kB nuclear factor kappa‐B, NLR family pyrin domain containing 3, PAMP pathogen associated molecular pattern
l-lactate, ROS and mitochondria: role in skin aging
l-lactate and mitochondria
During aging, the role of mitochondria in skin physiology is particularly crucial (Stout and Birch-Machin 2019; Sreedhar et al. 2020). Many authors agree with the idea that aging involves immunity in a process known as “inflammaging” (Franceschi et al. 2007). Mitochondria are a sort of “powerhouses” of immunity, as mitochondrial DNA (mtDNA) may act as a danger-associated molecular pattern (DAMP) and the same outer membrane of these organelles act as a bench for signaling components such as RIG-1 and MAVS and can activate also NLRP3 (inflammasome) (Mills et al. 2017). Circulating mtDNA correlates with increased Body Mass Index (BMI) and aging (Padilla-Sánchez et al. 2020) and has been since years considered a major biomarker of inflammation and cancer (Yu 2012). A mitochondrial functional biomarker is therefore associated with the presence of mitochondria genomes in the cell. Actually, the mitochondrial DNA copy number (mt-DNA CN), an evaluation of the number of the organelle genomes per cell, is used as a functional biomarker associated with aging-related disorders (Longchamps et al. 2020). Furthermore, during aging mitochondria DNA mutations cumulate and respiratory function declines (Wei et al. 2009). Mitochondria functionality is so crucial for skin health during aging that some authors suggested very recently an artificial mitochondria transfer/transplant (AMT/T) to promote renewal of senescent skin cells, even revitalizing them (Balcázar et al. 2020).
The relationship between l-lactate and “mitochondria health” may be closely related to aging processes. Some cognitive and neurological disorders links mtDNA alterations with increase in circulating l-lactate (Valiente-Pallejà et al. 2020; Hanisch et al. 2006). The importance of l-lactate for mitochondria bioenergetics has emerged very recently; for example, Young and colleagues showed that l-lactate can support fueling mouse mitochondria energetics, in liver, heart and muscle, via mitochondrial LDH, further participating in ROS generation and in the production of H2O2 to an extent comparable to pyruvate (Young et al. 2020). If l-lactate is fundamental for mitochondria, the “shuttle hypothesis” formulated by Brooks some years ago may suggest that l-lactate is used also as a signaling molecule to connect bioenergetics with cellular turnover (Brooks 2009). This hypothesis may find a confirmation as l-lactate oxidized in mitochondria exceeds of 10–40% the oxidation of pyruvate for bioenergetics (Brooks 2009).
The role of l-lactate in mitochondria biology is fundamental at least because the metabolism of l-lactate occurs in mitochondria (Chen et al. 2016; Glancy et al. 2021). Moreover, the relationship between mitochondria and aging dates back to the sixties and is certainly a major issue to elucidate the possible role of l-lactate and mitochondria in skin aging (Rockstein and Brandt 1963; Sun et al. 2016). The majority of studies on the relationship between mitochondria dysfunction and mtDNA mutations leading to the aged phenotype have been obtained by the so called “mitochondrial mutator mouse”, a knock out laboratory animal a mutated D257A gene, a proofreading deficient form of the polymerase POLGg, acting on mtDNA.
In these mice the gene encoded by the nucleus is the only mtDNA polymerase, which, as mutated at the position 257, lacks of the proofreading ability. Mice with one or two copies of this mutated gene, accumulated a huge deal of deficient mitochondria and show an accelerated senescent phenotype respect to wild type (Kujoth et al. 2005; Trifunovic et al. 2004). So far, the relationship between aging, mitochondria and l-lactate, has been particularly highlighted in molecular neuroscience (Datta and Chakrabarti 2018) but it can be speculatively suggested that a major role of l-lactate in modulating and regulating mitochondria-addressed aging may be retrieved from many other tissue and organ models, such as skin, where the dynamics of mitochondria and their intracellular connections is fundamental (Mellem et al. 2017). In mitochondria biogenesis the transcription co-activator factor peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α), is a master tuner of bioenergetics (Liang and Ward 2006). As a matter of fact, PGC-1α controls also l-lactate metabolism, for example by increasing the expression of MCT1, more than MCT2 and MCT4 in the skeletal muscle (Benton et al. 2008) and controlling the whole availability of l-lactate in the tissue (Summermatter et al. 2013). The administration of l-lactate increases the expression of PGC-1α, by inducing an enhancement in PGC-1α mRNA transcripts, and at the same time increases also pyruvate dehydrogenase kinase 4 (PDK4) and the mitochondrial uncoupling protein 3 (UCP3) gene expression (Kitaoka et al. 2016).
It is widely known that UCP3 should protect mitochondria from aging and also from inflammaging and fat-induced damage, via ROS as signaling molecules. Some authors, using C57Bl6 mice overexpressing skeletal muscle UCP3 (UCP3Tg), reported that 4-hydroxynonenal (4-HNE), an α-β unsaturated hydroxy-alchenal from lipidic peroxidation, dampens the age-related increase in ROS, due to the increased state IV of oxidative respiration from mitochondria by the UCP3 overexpression (Nabben et al. 2008). It is intriguing that 4-HNE is the major byproduct by oxygen-ozone treatment, which has been recently used even to improve the anti-inflammatory therapy against COVID-19 (Chirumbolo et al. 2021). The relationship between PGC-1α and ROS in particularly intriguing during the biogenesis of new mitochondria and mitophagy, where PGC-1α buffers the excess of ROS by eliciting the production of anti-oxidant scavenging enzymes (Baldelli et al. 2014). Mitochondria biogenesis and mitophagy are fundamental processes in counteracting aging (Wei et al. 2021; Bakula and Scheibye-Knudsen 2020; Chen et al. 2020). In this perspective, l-lactate may even have a crucial role.
The dynamin related protein 1 (DRP1), which regulates mitochondria biogenesis and mitophagy, i.e., mitochondrial and peroxisome fission, regulates also endoplasmic reticulum-generated droplets in the adipose tissue, so correlating the correct lipid storage in adipocytes with the mitochondrial survival (Li et al. 2020). Recent data on lung cancer cell models, reported that DRP1 promotes l-lactate utilization and suppresses oxidative stress from ROS (Hu et al. 2020). Targeting DRP1 allows to reducing the expression of heart shock proteins (hsp90) and increasing the ROS-mediated cleavage of hsp90, a mechanism that in turn will inhibit the MAPK and PI3K-mediated pathways, the ability of cell to use l-lactate and subsequently the ROS-mediated cell death (li et al. 2020).
Mitochondria fusion and fission are remarkable mechanisms in the development of the aging process, in which l-lactate would play a role (Liu et al. 2020a, b, c). Mitofusin-2, a protein of the outer membrane involved in mitochondria fusion, when deficient might regulate an adaptive response involving the increase in PGC-1α and in the transcription factor TFAM, to prevent mtDNA depletion (Kawalec et al. 2015). The bulk of evidence relating l-lactate with mitochondria and ROS strongly suggests that l-lactate, therefore, is intertwined with mitochondria turn over and probably with the molecular mechanisms leading to aging.
Mitochondria, l-lactate and aged skin
The role of mitochondria in skin aging has been recently reviewed (Stout and Birch-Machin 2019; Hudson et al. 2016). Noteworthy, mitochondrial metabolism is a leading process for keratinocyte differentiation. Deletion of TFAM at the level of basal cells of epidermis causes a loss in ROS signaling (reduction in the mitochondria production of ROS) and therefore an impairment in epidermal differentiation and also hair growth (Hamanaka and Chandel 2013). ROS are fundamental elements of tissue renewal and differentiation in the skin, including dermal compartments and adipose tissue (Rigotti and Chirumbolo 2019). Therefore, if ROS controls many fundamental aspects of skin biology, l-lactate, which is intertwined with the mitochondria-ROS signaling machinery, is of the utmost interest for skin differentiation and aging.
If mitochondria play an utmost role in skin differentiation, their involvement in aged skin should be particularly crucial.
Aged skin shows marked deterioration in mitochondria structure, cristae numbers, mtDNA copy number and mutations and defects in mitochondria biogenesis, fission and fusion, a process observed in many other epithelial tissues (Schneider et al. 2020). Recent reports showed that ubiquinol (reduced coenzyme Q10) has a powerful anti-aging effect on human dermal fibroblasts (Marcheggiani et al. 2021) and is a metabolic resuscitator in post-cardiac arrest (Holmberg et al. 2021). The production od ROS is fundamental for propagating both Notch and β-catenin, signals that are very strategic for epidermis differentiation and hair follicle development (Marcheggiani et al. 2021). Genetic defects in Coenzyme Q10 (CoQ10) biosynthetic pathway may affect l-lactate regulation, as the CoQ10 deficiency, which may be caused by a homozygous stop variant in COQ9 c.730C > T, pArg244*, should lead to neonatal lactic acidosis, general development delay and intractable seizures (Duncan et al. 2009).
As noted earlier, l-lactate protects skin fibroblasts via mito-hormesis (Zelenka et al. 2015). Previous literature has reported that l-lactate treatment upregulates the production of ROS as signaling molecules (Hashimoto et al. 2007), activates mitochondrial biogenesis via PGC-1α (Roland et al. 2014; Lezi et al. 2013) and stabilizes HIF-1α (Sonveaux et al. 2012). During aging, skin undergoes a complex plethora of biochemical and structural changes. For example, the role of NAD+-dependent deacetylases, known as sirtuins (SIRT 1–7), is fundamental to comprehend skin aging, particularly for photoaging, and the involvement of mitochondria biology (Su et al. 2020). Sirtuins 3 (SIRT3), 4 (SIRT4) and 5 (SIRT5), which are localized in mitochondria, are implicated in aging, besides to oxidative stress response and caloric restrictions (Gambini et al. 2011). Particularly for SIRT3 and SIRT4, their role is crucial for keratinocyte differentiation and wound repair (Su et al. 2020). Particularly for SIRT3, recent studies have reported that this deacetylase is a shield against aging and mitochondrial meltdown (Kincaid and Bossy-Wetzel 2013). Recent data reported that SIRT3 controls cell proliferation and glucose uptake overexpression (Cui et al. 2015). The majority of evidence regarding the role of sirtuins in the interplay cell metabolism-cell survival and proliferation/differentiation come from cancer studies (Gaál and Csernoch 2020), but SIRT3 is downregulated in lung idiopathic pneumonia with fibrosis (Rehan et al. 2021), where l-lactate is particularly elevated (Kottmann et al. 2012). It is possible to speculate that l-lactate is an alarming signal of a downregulation or impairment in the correct mitochondrial function and ROS availability as signaling molecules.
l-lactate, which is a molecular and metabolic starter to produce ROS as signaling molecules, is able to induce collagen synthesis and VEGF in endothelial cells (Zieker et al. 2009). Its action of collagen has been investigated in the past, when some authors discovered that l-lactate is one of the most powerful activator of collagen formation. By investigating L-929 fibroblasts Comstock and Udenfriend (1970) provided evidence confirmed by others with myofibroblasts and acetaldehyde, that l-lactate caused a significant (p < 0.02) enhancement in intracellular proline pool and collagen synthesis (Savolainen et al. 1984).
A fundamental role of l-lactate in reverting the aging process and promote the survival machinery of genes leading to tissue renewal, might be exerted on HO-1. This fundamentally occurs because HO-1, particularly skeletal HO-1, controls aerobic capacity, i.e. that the deletion in mice of the muscle-specific HO-1, in the transgenic mouse Tam-Cre-HSA-Hmox1fl/fl, changes the rate type IIA to type IIB muscle fibers, alongside with an overall disruption of mitochondria (Alves de Souza et al. 2021).
The induction of HO-1 under oxidative stress is mainly activated by the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which is regulated by the mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3k)/Akt, and protein kinase C (PKC) signaling pathways (Feng et al. 2017).
Conclusions
Aging in the skin is a major process where the activity of l-lactate as a signaling molecule might provide biomedical research with heuristically valid insights, particularly about the role of this metabolite in lowering senescence in the skin and to elucidate further the mechanisms underlying skin aging.
In this review we have fundamentally highlighted that:
-
l-lactate is a major signaling molecule able to interact with mitochondria biogenesis and turnover and tune the ability of cells to respond to stressors;
-
The fundamental way by which l-lactate works as a signaling molecule involve mechanisms known as “mito-hormesis”
-
Despite its nature of apparently useless byproduct, l-lactate plays fundamental roles in signaling of brain development related with exercise and in immune regulation;
-
Its role in skin rejuvenation and aging control can be supported by the survival machinery led by mitochondrial biogenesis.
The role of l-lactate should be included in the wider role of small molecules working as signaling compounds, able to finely regulate cell survival, cycle and development. In this sense, the importance to deepen the activity and the biological meaning of this glycolysis byproduct is paramount.
Data availability
Not applicable. No data are reported in this manuscript.
References
Adelsmayr G, Brunner R, Holzinger U (2012) Impact of blood glucose on blood lactate levels in a medical ICU: a retrospective cohort study. Crit Care 16(Suppl 1):P165
Alves de Souza RW, Gallo D, Lee GR, Katsuyama E, Schaufler A, Weber J, Csizmadia E, Tsokos GC, Koch LG, Britton SL, Wisløff U, Brum PC, Otterbein LE (2021) Skeletal muscle heme oxygenase-1 activity regulates aerobic capacity. Cell Rep 35(3):109018
Atayik MC, Çakatay U (2022) Mitochondria-targeted senotherapeutic interventions. Biogerontology 23(4):401–423
Bakula D, Scheibye-Knudsen M (2020) MitophAging: mitophagy in aging and disease. Front Cell Dev Biol 8:239
Baldelli S, Aquilano K, Ciriolo MR (2014) PGC-1α buffers ROS-mediated removal of mitochondria during myogenesis. Cell Death Dis 5(11):e1515
Balcázar M, Cañizares S, Borja T, Pontón P, Bisiou S, Carabasse E, Bacilieri A, Canavese C, Diaz RF, Cabrera F, Caicedo A (2020) Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T). Front Bioeng Biotechnol 8:919
Baltazar F, Afonso J, Costa M, Granja S (2020) Lactate beyond a waste metabolite: metabolic affairs and signaling in malignancy. Front Oncol 10:231
Barbosa MC, Grosso RA, Fader CM (2019) Hallmarks of aging: an autophagic perspective. Front Endocrinol (lausanne) 9:790
Bárcena C, Mayoral P, Quirós PM (2018) Mitohormesis, an antiaging paradigm. Int Rev Cell Mol Biol 340:35–77
Barzegari A, Aaboulhassanzadeh S, Landon R, Gueguen V, Meddahi-Pellé A, Parvizpour S, Anagnostou F, Pavon-Djavid G (2022) Mitohormesis and mitochondrial dynamics in the regulation of stem cell fate. J Cell Physiol 237(9):3435–3448
Benton CR, Yoshida Y, Lally J, Han XX, Hatta H, Bonen A (2008) PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genom 35(1):45–54
Bordon Y (2021) Protect the species with mitohormesis? Nat Rev Immunol 21(7):407
Brandi J, Cheri S, Manfredi M, Di Carlo C, Vita Vanella V, Federici F, Bombiero E, Bazaj A, Rizzi E, Manna L, Cornaglia G, Marini U, Valenti MT, Marengo E, Cecconi D (2020) Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci Rep 10(1):11572
Brisson L, Bański P, Sboarina M, Dethier C, Danhier P, Fontenille MJ, Van Hée VF, Vazeille T, Tardy M, Falces J, Bouzin C, Porporato PE, Frédérick R, Michiels C, Copetti T, Sonveaux P (2016) Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell 30(3):418–431
Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587(Pt 23):5591–5600
Brooks GA (2020) Lactate as a fulcrum of metabolism. Redox Biol 35:101454
Brouwers MC, Ham JC, Wisse E, Misra S, Landewe S, Rosenthal M, Patel D, Oliver N, Bilo HJ, Murphy E (2015) Elevated lactate levels in patients with poorly regulated type 1 diabetes and glycogenic hepatopathy: a new feature of Mauriac syndrome. Diabetes Care 38(2):e11–e12
Cai M, Wang H, Song H, Yang R, Wang L, Xue X, Sun W, Hu J (2022) Lactate is answerable for brain function and treating brain diseases: energy substrates and signal molecule. Front Nutr 9:800901
Chen YJ, Mahieu NG, Huang X, Singh M, Crawford PA, Johnson SL, Gross RW, Schaefer J, Patti GJ (2016) Lactate metabolism is associated with mammalian mitochondria. Nat Chem Biol 12(11):937–943
Chen G, Kroemer G, Mitophagy KO (2020) An emerging role in aging and age-associated diseases. Front Cell Dev Biol 8:200
Chirumbolo S, Valdenassi L, Simonetti V, Bertossi D, Ricevuti G, Franzini M, Pandolfi S (2021) Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int Immunopharmacol 96:107777
Coggan JS, Keller D, Markram H, Schürmann F, Magistretti PJ (2022) Representing stimulus information in an energy metabolism pathway. J Theor Biol 540:111090
Comstock JP, Udenfriend S (1970) Effect of lactate on collagen proline hydroxylase activity in cultured L-929 fibroblasts. Proc Natl Acad Sci USA 66(2):552–557
Cui Y, Qin L, Wu J, Qu X, Hou C, Sun W, Li S, Vaughan AT, Li JJ, Liu J (2015) SIRT3 enhances glycolysis and proliferation in SIRT3-expressing gastric cancer cells. PLoS ONE 10(6):e0129834
Danby FW (2010) Nutrition and aging skin: sugar and glycation. Clin Dermatol 28(4):409–411. https://doi.org/10.1016/j.clindermatol.2010.03.018
D’Autréaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8(10):813–824
Das CK, Parekh A, Parida PK, Bhutia SK, Mandal M (2019) Lactate dehydrogenase A regulates autophagy and tamoxifen resistance in breast cancer. Biochim Biophys Acta Mol Cell Res 1866(6):1004–1018
Datta S, Chakrabarti N (2018) Age related rise in lactate and its correlation with lactate dehydrogenase (LDH) status in post-mitochondrial fractions isolated from different regions of brain in mice. Neurochem Int 118:23–33
Di Marzo N, Chisci E, Giovannoni R (2018) The role of hydrogen peroxide in redox-dependent signaling: homeostatic and pathological responses in mammalian cells. Cells 7(10):156
Duda I, Krzych Ł, Jędrzejowska-Szypułka H, Lewin-Kowalik J (2020) Plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in critically ill patients. Open Med (wars) 15:50–56
Duncan AJ, Bitner-Glindzicz M, Meunier B et al (2009) A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet 84:558–566
Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos NT (2017) Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J 14(1):89–96
Durante W, Johnson FK, Johnson RA (2006) Role of carbon monoxide in cardiovascular function. J Cell Mol Med 10(3):672–686
Eckhart L, Tschachler E, Gruber F (2019) Autophagic control of skin aging. Front Cell Dev Biol 7:143
Eltzschig HK (2013) Extracellular adenosine signaling in molecular medicine. J Mol Med (berl) 91(2):141–146
Feng XE, Liang TG, Gao J, Kong P, Ge R, Li QS (2017) Heme oxygenase-1, a Key enzyme for the cytoprotective actions of halophenols by upregulating Nrf2 expression via activating Erk1/2 and PI3K/Akt in EA.hy926 cells. Oxid Med Cell Longev 2017:7028478
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49(5):835–842
Forsyth JJ, Reilly T (2004) Circadian rhythms in blood lactate concentration during incremental ergometer rowing. Eur J Appl Physiol 92(1–2):69–74
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S (2007) Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128(1):92–105
Freitas-Rodríguez S, Folgueras AR, López-Otín C (2017) The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res 1864(11 Pt A):2015–2025
Gaál Z, Csernoch L (2020) Impact of sirtuin enzymes on the altered metabolic phenotype of malignantly transformed cells. Front Oncol 10:45
Gambini J, Gomez-Cabrera MC, Borras C, Valles SL, Lopez-Grueso R, Martinez-Bello VE, Herranz D, Pallardo FV, Tresguerres JA, Serrano M, Viña J (2011) Free [NADH]/[NAD(+)] regulates sirtuin expression. Arch Biochem Biophys 512(1):24–29
Gauron C, Rampon C, Bouzaffour M, Ipendey E, Teillon J, Volovitch M, Vriz S (2013) Sustained production of ROS triggers compensatory proliferation and is required for regeneration to proceed. Sci Rep 3:2084
Glancy B, Kane DA, Kavazis AN, Goodwin ML, Willis WT, Gladden LB (2021) Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol 599(3):863–888
Go S, Kramer TT, Verhoeven AJ, Oude Elferink RPJ, Chang JC (2021) The extracellular lactate-to-pyruvate ratio modulates the sensitivity to oxidative stress-induced apoptosis via the cytosolic NADH/NAD+ redox state. Apoptosis 26(1–2):38–51
Guerrero-Morín JG, Santillán M (2020) Crosstalk dynamics between the circadian clock and the mTORC1 pathway. J Theor Biol 501:110360
Gunin AG, Petrov VV, Golubtzova NN, Vasilieva OV, Kornilova NK (2014) Age-related changes in angiogenesis in human dermis. Exp Gerontol 55:143–151
Haake AR, Roublevskaia I, Cooklis M (1998) Apoptosis: a role in skin aging? J Investig Dermatol Symp Proc 3(1):28–35
Hamanaka RB, Chandel NS (2013) Mitochondrial metabolism as a regulator of keratinocyte differentiation. Cell Logist 3(1):e25456
Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, Chandel NS (2013) Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal 6(261):ra8
Hanisch F, Müller T, Muser A, Deschauer M, Zierz S (2006) Lactate increase and oxygen desaturation in mitochondrial disorders–evaluation of two diagnostic screening protocols. J Neurol 253(4):417–423
Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA (2007) Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 21(10):2602–2612. https://doi.org/10.1096/fj.07-8174com
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B (2019) MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int 2019:7132708
Harman D (1972) Free radical theory of aging: dietary implications. Am J Clin Nutr 25(8):839–843
Holmberg MJ, Andersen LW, Moskowitz A, Berg KM, Cocchi MN, Chase M, Liu X, Kuhn DM, Grossestreuer AV, Hoeyer-Nielsen AK, Kirkegaard H, Donnino MW (2021) Ubiquinol (reduced coenzyme Q10) as a metabolic resuscitator in post-cardiac arrest: a randomized, double-blind, placebo-controlled trial. Resuscitation 162:388–395
Hu M, Zhao Y, Cao Y, Tang Q, Feng Z, Ni J, Zhou X (2020) DRP1 promotes lactate utilization in KRAS-mutant non-small-cell lung cancer cells. Cancer Sci 111(10):3588–3599
Huang HC, Lee IJ, Huang C, Chang TM (2020) Lactic acid bacteria and lactic acid for skin health and melanogenesis inhibition. Curr Pharm Biotechnol 21(7):566–577
Huang Z, Zhang Y, Zhou R, Yang L, Pan H (2021) Lactate as potential mediators for exercise-induced positive effects on neuroplasticity and cerebrovascular plasticity. Front Physiol 12:656455
Hudson L, Bowman A, Rashdan E, Birch-Machin MA (2016) Mitochondrial damage and ageing using skin as a model organ. Maturitas 93:34–40
Jang JY, Blum A, Liu J, Finkel T (2018) The role of mitochondria in aging. J Clin Invest 128(9):3662–3670
Jenkins G (2002) Molecular mechanisms of skin ageing. Mech Ageing Dev 123(7):801–810
Jiang Y, Mishima H, Sakai S, Liu YK, Ohyabu Y, Uemura T (2008) Gene expression analysis of major lineage-defining factors in human bone marrow cells: effect of aging, gender, and age-related disorders. J Orthop Res 26(7):910–917
Kawalec M, Boratyńska-Jasińska A, Beręsewicz M, Dymkowska D, Zabłocki K, Zabłocka B (2015) Mitofusin 2 deficiency affects energy metabolism and mitochondrial biogenesis in MEF cells. PLoS ONE 10(7):e0134162
Kim SY, Nair MG (2019) Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 97(3):258–267
Kim HP, Ryter SW, Choi AM (2006) CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol 46:411–449
Kitada M, Koya D (2021) Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. https://doi.org/10.1038/s41574-021-00551-9
Kitaoka Y, Takeda K, Tamura Y, Hatta H (2016) Lactate administration increases mRNA expression of PGC-1α and UCP3 in mouse skeletal muscle. Appl Physiol Nutr Metab 41(6):695–698
Klaus S, Ost M (2020) Mitochondrial uncoupling and longevity—a role for mitokines? Exp Gerontol 130:110796
Kohl E, Steinbauer J, Landthaler M, Szeimies RM (2011) Skin ageing. J Eur Acad Dermatol Venereol 25(8):873–884
Kozlov AM, Lone A, Betts DH, Cumming RC (2020) Lactate preconditioning promotes a HIF-1α-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci Rep 10(1):8388
Kruk J, Aboul-Enein HY, Kładna A, Bowser JE (2019) Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radic Res 53(5):497–521
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309(5733):481–484
Kida YS, Kawamura T, Wei Z, Sogo T, Jacinto S, Shigeno A, Kushige H, Yoshihara E, Liddle C, Ecker JR, Yu RT, Atkins AR, Downes M, Evans RM (2015) ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell 16(5):547–555
Kincaid B, Bossy-Wetzel E (2013) Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 5:48
Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, Nathan SD, Grant G, Phipps RP, Sime PJ (2012) Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med 186(8):740–751
Kwon TG, Zhao X, Yang Q, Li Y, Ge C, Zhao G, Franceschi RT (2011) Physical and functional interactions between Runx2 and HIF-1α induce vascular endothelial growth factor gene expression. J Cell Biochem 112(12):3582–3593
Lago JC, Puzzi MB (2019) The effect of aging in primary human dermal fibroblasts. PLoS ONE 14(7):e0219165
Lafita-Navarro MC, Perez-Castro L, Zacharias LG, Barnes S, DeBerardinis RJ, Conacci-Sorrell M (2020) The transcription factors aryl hydrocarbon receptor and MYC cooperate in the regulation of cellular metabolism. J Biol Chem 295(35):12398–12407
Larsson NG (2010) Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem 79:683–706
Lee TY (2021) Lactate: a multifunctional signaling molecule. Yeungnam Univ J Med 38(3):183–193
Lee DE, Ayoub N, Agrawal DK (2016) Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 7:37. https://doi.org/10.1186/s13287-016-0303-6
Levasseur JE, Alessandri B, Reinert M, Clausen T, Zhou Z, Altememi N, Bullock MR (2006) Lactate, not glucose, up-regulates mitochondrial oxygen consumption both in sham and lateral fluid percussed rat brains. Neurosurgery 59(5):1122–1130 (discussion 1130-1)
Lezi E, Lu J, Selfridge JE, Burns JM, Swerdlow RH (2013) Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 127(1):91–100
Li X, Yang L, Mao Z, Pan X, Zhao Y, Gu X, Eckel-Mahan K, Zuo Z, Tong Q, Hartig SM, Cheng X, Du G, Moore DD, Bellen HJ, Sesaki H, Sun K (2020) Novel role of dynamin-related-protein 1 in dynamics of ER-lipid droplets in adipose tissue. FASEB J 34(6):8265–8282
Liang H, Ward WF (2006) PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ 30(4):145–151
Liemburg-Apers DC, Willems PH, Koopman WJ, Grefte S (2015) Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol 89(8):1209–1226
Liu YJ, McIntyre RL, Janssens GE, Houtkooper RH (2020a) Mitochondrial fission and fusion: a dynamic role in aging and potential target for age-related disease. Mech Ageing Dev 186:111212
Liu J, Ding Y, Liu Z, Liang X (2020b) Senescence in mesenchymal stem cells: functional alterations, molecular mechanisms, and rejuvenation strategies. Front Cell Dev Biol 8:258
Liu J, Chen S, Biswas S, Nagrani N, Chu Y, Chakrabarti S, Feng B (2020c) Glucose-induced oxidative stress and accelerated aging in endothelial cells are mediated by the depletion of mitochondrial SIRTs. Physiol Rep 8(3):e14331. https://doi.org/10.14814/phy2.14331
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772
Lisowski P, Kannan P, Mlody B, Prigione A (2018) Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep 19(5):e45432
Longchamps RJ, Castellani CA, Yang SY, Newcomb CE, Sumpter JA, Lane J, Grove ML, Guallar E, Pankratz N, Taylor KD, Rotter JI, Boerwinkle E, Arking DE (2020) Evaluation of mitochondrial DNA copy number estimation techniques. PLoS ONE 15(1):e0228166
Mahbub S, Deburghgraeve CR, Kovacs EJ (2012) Advanced age impairs macrophage polarization. J Interferon Cytokine Res 32(1):18–26
Manojlović V, Erčulj F (2019) Using blood lactate concentration to predict muscle damage and jump performance response to maximal stretch-shortening cycle exercise. J Sports Med Phys Fit 59(4):581–586
Manosalva C, Quiroga J, Hidalgo AI, Alarcón P, Anseoleaga N, Hidalgo MA, Burgos RA (2022) Role of lactate in inflammatory processes: friend or foe. Front Immunol 12:808799
Mann BE (2010) Carbon monoxide: an essential signalling molecule. In: Jaouen G, Metzler-Nolte N (eds) Medicinal organometallic chemistry. Topics in organometallic chemistry, vol 32. Springer, Berlin. https://doi.org/10.1007/978-3-642-13185-1_10
Marcheggiani F, Kordes S, Cirilli I, Orlando P, Silvestri S, Vogelsang A, Möller N, Blatt T, Weise JM, Damiani E, Tiano L (2021) Anti-ageing effects of ubiquinone and ubiquinol in a senescence model of human dermal fibroblasts. Free Radic Biol Med 165:282–288
Matsuo T, Daishaku S, Sadzuka Y (2019) Lactic acid promotes cell survival by blocking autophagy of B16F10 mouse melanoma cells under glucose deprivation and hypoxic conditions. Biol Pharm Bull 42(5):837–839
Mellem D, Sattler M, Pagel-Wolff S, Jaspers S, Wenck H, Rübhausen MA, Fischer F (2017) Fragmentation of the mitochondrial network in skin in vivo. PLoS ONE 12(6):e0174469
Merry TL, Ristow M (2016) Mitohormesis in exercise training. Free Radic Biol Med 98:123–130
Mills EL, Kelly B, O’Neill LAJ (2017) Mitochondria are the powerhouses of immunity. Nat Immunol 18(5):488–498
Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S (2013) High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE 8(1):e54514
Mosienko V, Teschemacher AG, Kasparov S (2015) Is L-lactate a novel signaling molecule in the brain? J Cereb Blood Flow Metab 35(7):1069–1075
Müller P, Duderstadt Y, Lessmann V, Müller NG (2020) Lactate and BDNF: key mediators of exercise induced neuroplasticity? J Clin Med 9(4):1136
Musci RV, Hamilton KL, Linden MA (2019) Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension. Sports (basel) 7(7):170
Nabben M, Hoeks J, Briedé JJ, Glatz JF, Moonen-Kornips E, Hesselink MK, Schrauwen P (2008) The effect of UCP3 overexpression on mitochondrial ROS production in skeletal muscle of young versus aged mice. FEBS Lett 582(30):4147–4152
Nalbandian M, Takeda M (2016) Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology (basel) 5(4):38
Ndiaye MA, Nihal M, Wood GS, Ahmad N (2014) Skin, reactive oxygen species, and circadian clocks. Antioxid Redox Signal 20(18):2982–2996
Nikooie R, Moflehi D, Zand S (2021) Lactate regulates autophagy through ROS-mediated activation of ERK1/2/m-TOR/p-70S6K pathway in skeletal muscle. J Cell Commun Signal 15(1):107–123
Padilla-Sánchez SD, Navarrete D, Caicedo A, Teran E (2020) Circulating cell-free mitochondrial DNA levels correlate with body mass index and age. Biochim Biophys Acta Mol Basis Dis 1866(12):165963
Park HY, Kim JH, Jung M, Chung CH, Hasham R, Park CS, Choi EH (2011) A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp Dermatol 20(12):969–974
Pellerin L, Connes P, Bisbal C, Lambert K (2022) Editorial: lactate as a major signaling molecule for homeostasis. Front Physiol 13:910567
Philp A, Macdonald AL, Watt PW (2005) Lactate–a signal coordinating cell and systemic function. J Exp Biol 208(Pt 24):4561–4575
Prieto J, Seo AY, León M, Santacatterina F, Torresano L, Palomino-Schätzlein M, Giménez K, Vallet-Sánchez A, Ponsoda X, Pineda-Lucena A, Cuezva JM, Lippincott-Schwartz J, Torres J (2018) MYC induces a hybrid energetics program early in cell reprogramming. Stem Cell Rep 11(6):1479–1492
Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, Blümlein K, Wanker EE, Ralser M, Cramer T, Adjaye J (2014) HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells 32(2):364–376
Ratter JM, Rooijackers HMM, Hooiveld GJ, Hijmans AGM, de Galan BE, Tack CJ, Stienstra R (2018) In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front Immunol 9:2564
Rehan M, Kurundkar D, Kurundkar AR, Logsdon NJ, Smith SR, Chanda D, Bernard K, Sanders YY, Deshane JS, Dsouza KG, Rangarajan S, Zmijewski JW, Thannickal VJ (2021) Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging 1(2):205–217
Reilly T, Waterhouse J (2009) Sports performance: is there evidence that the body clock plays a role? Eur J Appl Physiol 106(3):321–332
Reinke H, Asher G (2019) Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol 20(4):227–241
Rezvani HR, Ali N, Serrano-Sanchez M, Dubus P, Varon C, Ged C, Pain C, Cario-André M, Seneschal J, Taïeb A, de Verneuil H, Mazurier F (2011) Loss of epidermal hypoxia-inducible factor-1α accelerates epidermal aging and affects re-epithelialization in human and mouse. J Cell Sci 124(Pt 24):4172–4183
Rigotti G, Chirumbolo S (2019) Biological morphogenetic surgery: a minimally invasive procedure to address different biological mechanisms. Aesthet Surg J 39(7):745–755
Rockstein M, Brandt KF (1963) Enzyme changes in flight muscle correlated with aging and flight ability in the male housefly. Science 139(3559):1049–1051
Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB (2015) Lactate is always the end product of glycolysis. Front Neurosci 9:22
Roger F, Picazo C, Reiter W, Libiad M, Asami C, Hanzén S, Gao C, Lagniel G, Welkenhuysen N, Labarre J, Nyström T, Grøtli M, Hartl M, Toledano MB, Molin M (2020) Peroxiredoxin promotes longevity and H2O2-resistance in yeast through redox-modulation of protein kinase A. Elife 9:e60346
Roland CL, Arumugam T, Deng D, Liu SH, Philip B, Gomez S, Burns WR, Ramachandran V, Wang H, Cruz-Monserrate Z, Logsdon CD (2014) Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res 74(18):5301–5310
Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, Larsson NG, Hoffer BJ, Olson L (2010) High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci USA 107(46):20087–20092
Savolainen ER, Leo MA, Timpl R, Lieber CS (1984) Acetaldehyde and lactate stimulate collagen synthesis of cultured baboon liver myofibroblasts. Gastroenterology 87(4):777–787
Schmidt A, Karlson-Stiber C (2008) Caffeine poisoning and lactate rise: an overlooked toxic effect? Acta Anaesthesiol Scand 52(7):1012–1014
Schneider AM, Özsoy M, Zimmermann FA, Feichtinger RG, Mayr JA, Kofler B, Sperl W, Weghuber D, Mörwald K (2020) Age-related deterioration of mitochondrial function in the intestine. Oxid Med Cell Longev 2020:4898217
Seheult J, Fitzpatrick G, Boran G (2017) Lactic acidosis: an update. Clin Chem Lab Med 55(3):322–333
Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, Jin P, Bazin R, Patey N, Marincola FM, Moldovan F, Zaouter C, Trudeau LE, Benabdhalla B, Louis I, Beauséjour C, Stroncek D, Le Deist F, Haddad E (2016) Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget 7(21):30193–30210
Smith WP (1996) Epidermal and dermal effects of topical lactic acid. J Am Acad Dermatol 35(3 Pt 1):388–391
Song J, Lee K, Park SW, Chung H, Jung D, Na YR, Quan H, Cho CS, Che JH, Kim JH, Park JH, Seok SH (2018) Lactic acid upregulates VEGF expression in macrophages and facilitates choroidal neovascularization. Invest Ophthalmol vis Sci 59(8):3747–3754
Sonveaux P, Copetti T, De Saedeleer CJ, Végran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frérart F, Gallez B, Ribeiro A, Michiels C, Dewhirst MW, Feron O (2012) Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 7(3):e33418
Sreedhar A, Aguilera-Aguirre L, Singh KK (2020) Mitochondria in skin health, aging, and disease. Cell Death Dis 11(6):444
Srivastava S (2017) The mitochondrial basis of aging and age-related disorders. Genes (basel) 8(12):398
Stabenow LK, Zibrova D, Ender C, Helbing DL, Spengler K, Marx C, Wang ZQ, Heller R (2022) Oxidative glucose metabolism promotes senescence in vascular endothelial cells. Cells 11(14):2213
Stern R, Shuster S, Neudecker BA, Formby B (2002) Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res 276(1):24–31
Stout R, Birch-Machin M (2019) Mitochondria’s role in skin ageing. Biology (basel) 8(2):29
Su S, Ndiaye M, Singh CK, Ahmad N (2020) Mitochondrial sirtuins in skin and skin cancers. Photochem Photobiol 96(5):973–980
Summermatter S, Santos G, Pérez-Schindler J, Handschin C (2013) Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA 110(21):8738–8743
Sun N, Youle RJ, Finkel T (2016) The mitochondrial basis of aging. Mol Cell 61(5):654–666
Tower J (2015) Programmed cell death in aging. Ageing Res Rev 23(Pt A):90–100. https://doi.org/10.1016/j.arr.2015.04.002
Tauffenberger A, Fiumelli H, Almustafa S, Magistretti PJ (2019) Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis 10(9):653
Tran D, Townley JP, Barnes TM, Greive KA (2014) An antiaging skin care system containing alpha hydroxy acids and vitamins improves the biomechanical parameters of facial skin. Clin Cosmet Investig Dermatol 8:9–17
Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429(6990):417–423
Tuteja N, Chandra M, Tuteja R, Misra MK (2004) Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. J Biomed Biotechnol 2004(4):227–237
Unternaehrer JJ, Zhao R, Kim K, Cesana M, Powers JT, Ratanasirintrawoot S, Onder T, Shibue T, Weinberg RA, Daley GQ (2014) The epithelial-mesenchymal transition factor SNAIL paradoxically enhances reprogramming. Stem Cell Rep 3(5):691–698
Valiente-Pallejà A, Torrell H, Alonso Y, Vilella E, Muntané G, Martorell L (2020) Increased blood lactate levels during exercise and mitochondrial DNA alterations converge on mitochondrial dysfunction in schizophrenia. Schizophr Res 220:61–68
Van Hall G (2000) Lactate as a fuel for mitochondrial respiration. Acta Physiol Scand 168(4):643–656
van Vliet AR, Agostinis P (2018) Mitochondria-associated membranes and ER stress. Curr Top Microbiol Immunol 414:73–102
van Vliet AR, Verfaillie T, Agostinis P (2014) New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 1843(10):2253–2262
Veloz Castillo MF, Magistretti PJ, Calì C (2021) L-lactate: food for thoughts. Mem Behav Metab 11(8):548
Wallace NK, Pollard F, Savenkova M, Karatsoreos IN (2020) Effect of aging on daily rhythms of lactate metabolism in the medial prefrontal cortex of male mice. Neuroscience 448:300–310
Wagner S, Hussain MZ, Hunt TK, Bacic B, Becker HD (2004) Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Repair Regen 12(3):368–373
Wei YH, Wu SB, Ma YS, Lee HC (2009) Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging. Chang Gung Med J 32(2):113–132
Wei X, Wu YE, Wang W, Zhang S, Liu D, Liu H (2021) Decreased dynamin-related protein 1-related mitophagy induces myocardial apoptosis in the aging heart. Acta Biochim Biophys Sin (shanghai). https://doi.org/10.1093/abbs/gmab112
Wu Y, Ma W, Liu W, Zhang S (2022) Lactate: a pearl dropped in the ocean: an overlooked signal molecule in physiology and pathology. Cell Biol Int. https://doi.org/10.1002/cbin.11975
Yin M, Zhang Y, Yu H, Li X (2021) Role of hyperglycemia in the senescence of mesenchymal stem cells. Front Cell Dev Biol 9:665412
Young A, Oldford C, Mailloux RJ (2020) Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol 28:101339
Yu M (2012) Circulating cell-free mitochondrial DNA as a novel cancer biomarker: opportunities and challenges. Mitochondrial DNA 23(5):329–332
Zhang S, Duan E (2018) Fighting against skin aging: the way from bench to bedside. Cell Transplant 27(5):729–738
Zhang Y, Herman B (2002) Apoptosis and successful aging. Mech Ageing Dev 123(6):563–565
Zhang D, Lu H, Chen Z, Wang Y, Lin J, Xu S, Zhang C, Wang B, Yuan Z, Feng X, Jiang X, Pan J (2017) High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol Med Rep 16(2):1685–1690
Zhang H, Menzies KJ, Auwerx J (2018) The role of mitochondria in stem cell fate and aging. Development 145(8):dev143420
Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L, Zhao Y (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574(7779):575–580
Zelenka J, Dvořák A, Alán L (2015) L-lactate protects skin fibroblasts against aging-associated mitochondrial dysfunction via mitohormesis. Oxid Med Cell Longev 2015:351698
Zieker D, Küper M, Löffler M, Northoff H, Königsrainer A, Hunt TK, Beckert S, (2009) Lactate induces collagen synthesis, VEGF expression and migration in endothelial cells via generation of superoxide. In: Schumpelick V, Bruch HP, Schackert HK (eds) Chirurgisches forum und DGAV forum 2009. Deutsche Gesellschaft für Chirurgie, vol 38. Springer, Berlin. https://doi.org/10.1007/978-3-642-00625-8_110
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. No funding to be declared.
Author information
Authors and Affiliations
Contributions
This manuscript was completely conceived and written by SC. Prof. DB and Prof PM read the paper, suggested notes and revision, validated the final manuscript submitted. Therefore the paper was completely conceived by SC.
Corresponding author
Ethics declarations
Conflict of interest
The Authors state they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chirumbolo, S., Bertossi, D. & Magistretti, P. Insights on the role of l-lactate as a signaling molecule in skin aging. Biogerontology 24, 709–726 (2023). https://doi.org/10.1007/s10522-023-10018-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-023-10018-1