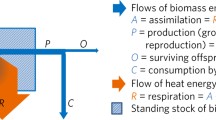
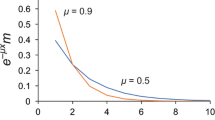
Abstract
Free-living organisms normally struggle to exist in harsh environments that are nutritionally and energetically inadequate, where evolutionary adaptation is challenged by internal stresses within organisms and external stresses from the environment. The incorporation of environmental variables into aging theories such as the free-radical and metabolic rate/oxidative stress theories, is the basis of the ecological stress theory of aging and hormesis. Environmental variation from optimum to lethal extremes gives a fitness-stress continuum, where energetic efficiency, or fitness, is inversely related to stress level; in the evolutionary context survival is a more direct measure of fitness for assessing aging than is lifespan. On this continuum, the hormetic zone is in the optimum region, while aging emphasizes survival towards lethal extremes. At the limits of survival, a convergence of physiological and genetical processes is expected under accumulating stress from Reactive Oxygen Species, ROS. Limited ecologically-oriented studies imply that major genes are important towards limits of survival compared with the hormetic zone. Future investigations could usefully explore outlier populations physiologically and genetically, since there is the likelihood that genetic variability may be lower in those cohorts managing to survive to extremely advanced ages as found in highly stressed ecological outlier populations. If so, an evolutionary explanation of the mortality-rate decline typical of cohorts of the extremely old emerges. In summary, an energetic evolutionary approach produces a general aging theory which automatically incorporates hormesis, since the theory is based on a fitness-stress continuum covering the whole range of possible abiotic environments of natural populations.
Similar content being viewed by others
References
Alonso-Alvarez C, Bertrand S, Deveney G, Prost J, Faivre B, Chastel O, Sorci G (2006) An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution 60:1913–1924
Ames BN (2004) Delaying the mitochondrial decay of aging. Ann NY Acad Sci 1019:406–411
Arking R (1998) Biology of aging: observations and principles, 2nd edn. Sinauer Associates, Sunderland, Massachusetts
Austad SN (1997) Why we age. John Wiley, New York
Barja G (2004) Aging in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production––DNA damage mechanism? Biol Rev 79:235–251
Bernstein AM, Willcox BJ, Tamaki H, Kunishima N, Suziki M, Willcox DC, Yoo JS, Perls TT (2004) First autopsy study of an Okinawan centenarian: absence of many age related diseases. J Gerontol A Biol Sci Med Sci 59:1195–1199
Boulétreau-Merle J, Fouillet P, Terrier O (1992) Clinical and seasonal variations in initial retention of virgin Drosophila melanogaster females as a strategy for fitness. Evol Ecol 6:223–242
Calabrese EJ (2006) The failure of dose-response models to predict low dose effects: a major challenge for biomedical, toxicological and aging research. Biogerontology 7:117–122
Calabrese EJ, Baldwin LA (2000) The effects of gamma rays on longevity. Biogerontology 1:309–319
Caratero A, Courtade M, Bennet L, Planel H, Caratero C (1998) Effect of a continuous gamma irradiation at a very low dose on the life span of mice. Gerontology 44:272–276
Carey J, Liedo P, Orozco D, Vaupel JW (1992) Slowing of mortality rates at older ages in large Medfly cohorts. Science 258:457–461
Carnes BA, Olshansky SJ, Grahn D (2003) Biological evidence for limits to the duration of life. Biogerontology 4:31–45
Curtsinger JW, Fukui RH, Townsend DR, Vaupel (1992) Demography of genotypes: failure of the limited life-span paradigm in Drosophila melanogaster. Science 258:461–462
Darviri C, Tigani X, Demakakos P (2006) Healthy longevity of Greek Centenarians: a quantitative and qualitative exploration of exceptional longevity. 3rd International Conference on Healthy Ageing and Longevity. Melbourne, Abstract p92
Demetrius L (2005) Of mice and men. EMBO Reports 6:S39–S44
Diamond JM, Hammond KA (1992) Intestinal determinants of muscle performance. Adv Biosciences 84:163–170
Dissanayake C (2005) Of stones and health: medical geology in Sri Lanka. Science 309:883–885
Dobzhansky T (1973) Nothing in biology makes sense except in the light of evolution. Am Biol Teach, March:125–129
Drenos F, Westendorp RGJ, Kirkwood TBL (2006) Trade-off mediated effects on the genetics of human survival caused by increasingly benign living conditions. Biogerontology 7:287–295
Falkowski PG (2006) Tracing oxygen’s imprint on Earth’s metabolic evolution. Science 311:1724–1725
Feinendegen LE (2005) Evidence for beneficial low level radiation effects and hormesis. Brit J Radiol 78:3–7
Giess M-C, Planel H (1973) Influence de la radioprotection effectué á différents stades sur la longévité de Drosophila melanogaster. CR Acad Sci Paris 276:1029–1032
Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH (2006) Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA 103:14158–14163
Heininger K (2001) The deprivation syndrome is the driving force of phylogeny, ontogeny and oncogeny. Rev Neurosciences 12:217–287
Hekimi S, Guarente L (2003) Genetics and the specificity of the aging process. Science 299:1351–1354
Hipkiss AR (2003) Errors, mitochondrial dysfunction and ageing. Biogerontology 4:397–400
Jazwinski SM (1996) Longevity, genes and aging. Science 273:55–59
Jenkins NL, McColl G, Lithgow GJ (2004)Fitness cost and extended lifespan in Caenorhabditis elegans. Proc Roy Soc Lond B 271:2523–2526
Kerber RA, Boucher KM, Cawthon RM, O’Brien E (2006) Gene expression profiles predicting life expectancy in humans. 3rd International Conference on Healthy Ageing and Longevity Melbourne. Abstract, pp 76–77
Kerber RA, O’Brien E, Smith KR, Cawthon RA (2001) Familial excess longevity in Utah genealogies. J Gerontol Biol Sci 56A:B130–B139
Kis-Papo T, Kirzhner V, Wasser SP, Nevo E (2003) Evolution of genomic diversity and sex at extreme environments. Fungal life under hypersaline Dead Sea stress. Proc Nat Acad Sci USA 100:14970–14975
Kowald A (2002) Lifespan does not measure ageing. Biogerontology 3:187–190
Lowenthal GC, Airey PL (2001) Practical applications of radioactivity and nuclear reactions. Cambridge University Press, Cambridge
MacDonald IF, Kempster B, Zanette L, MacDougall-Shackleton SA (2006) Early nutritional stress impairs development of a song-control brain region in both male and female juvenile song sparrows (Melospiza melodia) at the onset of song learning. Proc Roy Soc B 273:2559–2564
Matheson AC, Parsons PA (1973) The genetics of resistance to long-term exposure to CO2 in Drosophila melanogaster: an environmental stress leading to anoxia. Theoret Appl Genet 43:261–268
Meehan B, White NG (eds) (1990) Hunter-gatherer demography: past and present. University of Sydney, Sydney
Minois N (2000) Longevity and aging: beneficial effects of exposure to mild stress. Biogerontology 1:15–29
Miyazaki S, Nevo E, Grishkan I, Idleman U, Weinberg D, Bohnert HJ (2003) Oxidative stress responses in yeast strains, Saccharomyces cerevisiae, from ‘Evolution Canyon’, Israel. Monatschefte für Chemie 134:1465–1480
Nevo E (2001) Evolution of genome-phenome diversity under environmental stress. Proc Natl Acad Sci USA 98:6233–6240
Novoseltsev VN, Novoseltseva J, Yashin AI (2001) A homeostatic model of oxidative damage explains paradoxes observed in earlier experiments: a fusion and extension of older theories of aging. Biogerontology 2:127–138
Olsen A, Vantipalli MC, Lithgow GJ (2006) Lifespan extension of Caenorhabditis elegans following repeated mild hormetic treatments. Biogerontology 7:221–230
Olshansky SJ, Rattan SIS(2005) At the heart of aging: is it metabolic rate or stability? Biogerontology 6:291–295
Parsons PA (1974) Genetics of resistance to environmental stresses in Drosophila populations. Ann Rev Genet 7:239–265
Parsons PA (1982) Adaptive strategies of colonizing animal species. Biol Rev 57:117–148
Parsons PA (1992) Evolutionary adaptation and stress: the fitness gradient. Evol Biol 26:191–223
Parsons PA (1995) Inherited stress resistance and longevity: a stress theory of ageing. Heredity 75:216–221
Parsons PA (2002a) Radiation hormesis: challenging LNT theory via ecological and evolutionary considerations. Health Physics 82:513–516
Parsons PA (2002b) Life span: does the limit to survival depend upon metabolic efficiency under stress? Biogerontology 3:233–241
Parsons PA (2003) Energy, stress and the invalid linear no-threshold premise: a generalization illustrated by ionizing radiation. Biogerontology 4:227–231
Parsons PA (2004) From energy efficiency under stress to rapid development and a long life in natural populations. Biogerontology 5:201–210
Parsons PA (2005) Environments and evolution: interactions between stress, resource inadequacy and energetic efficiency. Biol Rev 80:589–610
Parsons PA (2006) Radiation, ecology and the invalid LNT model: the evolutionary imperative. Dose Response 4:191–200
Parsons PA (2007a) Survival and longevity improvements at extreme ages: an interpretation assuming an ecological stress theory of aging. Biogerontology 8: (in press)
Parsons PA (2007b) Energetic efficiency under stress underlies positive genetic correlations between longevity and other fitness traits in natural populations. Biogerontology 8:55–61
Planel H, Soleilhavoup JP, Tixador R, Richoilley G, Conter A, Croute F et al (1987) Influence on cell proliferation of background radiation or exposure to very low chronic gamma-radiation. Health Phys 52:571–578
Pollycove M, Feinendegen LE (2001) Biological responses to low doses of ionizing radiation: detriment versus hormesis Part 2. Dose responses of organisms. J Nuclear Medicine 42:26N–37N
Rattan SIS (2004) Aging, anti-aging and hormesis. Mech Ageing Dev 123:285–289
Raymond J, Segre D (2006) The effect of oxygen on biochemical networks and the evolution of complex life. Science 311:1764–1767
Rose MR, Rauser CL, Mueller LD, Benford G (2006) A revolution for aging research. Biogerontology 7:269–277
Rosewell J, Shorrocks B (1987) The implication of survival rates in natural populations of Drosophila: capture-recapture experiments on domestic species. Biol J Linn Soc 32:373–384
Snoke MS, Promislow DEL (2003) Quantitative genetic tests of recent senescence theory: age-specific mortality and male fertility in Drosophila melanogaster. Heredity 91:546–556
Tubiana M, Aurengo A. Averbeck D, Bonnin A, Le Guen B, Masse R, Monier R, Valleron AJ, de Vathairel F (2005) Dose-effect relationships and estimation of the carcinogenic effect of low doses of ionizing radiation. Académie des Sciences-Académie National de Médecine, Paris, 94 pp
Vaupel JW (1988) Inherited frailty and longevity. Demography 25:277–287
Vaupel JW (1997) The remarkable improvements in survival at older ages. Proc R Soc Lond B 352:1799–1804
Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV et al (1998) Biodemographic trajectories of longevity. Science 280:855–860
Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ (2000) Evolution of lifespan in C. elegans. Nature 405:296–297
Wang Y, Pot D, Kachman SD, Nuzhdin SV, Harshman LG (2006) A quantitative trait locus analysis of natural genetic variation for Drosophila melanogaster oxidative stress survival. J Heredity 97:355–366
Westerman JM, Parsons PA (1973) Variations in genetic architecture at different doses of γ-radiation as measured by longevity in Drosophila melanogaster. Can J Genet Cytol 15:289–298
White TCR (1993) The inadequate environment: nitrogen and the abundance of animals. Springer-Verlag, Berlin
Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M (2006a) Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology 7:173–177
Willcox BJ, Willcox DC, He Q, Curd JD, Suzuki M (2006b) Siblings of Okinawan centenarians share lifelong mortality advantages. J. Gerontol ABiol Sci Med Sci 61:345-
Yu BP, Chung HY (2001) Stress resistance by caloric restriction for longevity. NY Acad Sci 928:39–47
Zotin AI (1990) Thermodynamic bases of biological processes: physiological reactions and interactions. Walter de Gruyter, New York
Acknowledgements
I am grateful to Lee Ehrman for alerting me to some key references, and to Suresh Rattan for encouraging me to participate in the 3rd International Conference on Health Ageing and Longevity in Melbourne in late 2006, and to a reviewer whose comments improved the paper substantially.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parsons, P.A. The ecological stress theory of aging and hormesis: an energetic evolutionary model. Biogerontology 8, 233–242 (2007). https://doi.org/10.1007/s10522-007-9080-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-007-9080-z