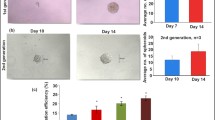
We studied the effects of secretory products of the placenta obtained from women with normal pregnancy and preeclampsia on the expression of surface markers by THP-1 cells cultured on a 3D Matrigel scaffold. Secretory products of third trimester placentas obtained from women with normal pregnancy reduced the relative number of THP-1 cells expressing CD54 and CD14 molecules and expression of CD14 and CD95 molecules by THP-1 cells in comparison with the effect of secretory products first trimester placentas. In parallel, the intensity of CD49d expression by THP-1 cells increased in the presence of secretory products of third trimester placentas in comparison with the first trimester. No differences in the expression of the studied molecules by THP-1 cells under the effect of placentas from women with physiological pregnancy and patients with preeclampsia were found.
Similar content being viewed by others
References
T. Yu. Lvova, Ya. S. Onochina, D. A. Korenkov, O. I. Stepanova, K. N. Furaeva, E. A. Potolicina, S. A. Selkov, and D. I. Sokolov, Influence of soluble placenta-derived factors upon expression of surface receptors on THP-1 cells. Med. Immunol., 15, No. 5, 449-456 (2013).
D. I. Sokolov and S. A. Sel’kov, Immunological Regulation of the Formation of the Vascular Network in the Placenta [in Russian], St. Petersburg (2012).
A. A. Totolyuan and I. S. Freidlin, Cells of the Immune System [in Russian] St. Petersburg (2000).
S. M. Albelda and C. A. Buck, Integrins and other cell adhesion molecules. FASEB J., 4, No. 11, 2868-2880 (1990).
R. S. Apte, Regulation of angiogenesis by macrophages. Adv. Exp. Med. Biol., 664, 15-19 (2010).
A. M. Borzychowski, I. L. Sargent, and C. W. Redman, Inflammation and pre-eclampsia. Semin. Fetal Neonatal Med., 11, No. 5, 309-316 (2006).
T. M. Carlos and J. M. Harlan, Leukocyte-endothelial adhesion molecules. Blood, 84, No. 7, 2068-2101 (1994).
S. Elmore, Apoptosis: a review of programmed cell death. Toxicol. Pathol., 35, No. 4, 495-516 (2007).
M. M. Faas, F Spaans., and P. De Vos, Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol., 5, 298, doi: 10.3389/fimmu.2014.00298 (2014).
S. S. Jacob, P. Shastry, and P. R. Sudhakaran, Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol. Cell. Biochem., 233, Nos. 1-2, 9-17 (2002).
P. Kaufmann, T. M. Mayhew, and D. S. Charnock-Jones, Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta, 25, No. 2-3, 114-126 (2004).
M. Miyazawa, Y. Ito, N. Kosaka, Y. Nukada, H. Sakaguchi, H. Suzuki, and N. Nishiyama, Role of TNF-alpha and extracellular ATP in THP-1 cell activation following allergen exposure. J. Toxicol. Sci., 33, No. 1, 71-83 (2008).
L. Moldovan and N. I. Moldovan, Role of monocytes and macrophages in angiogenesis. EXS, No. 94, 127-146 (2005).
G. Mor and V. M. Abrahams, Potential role of macrophages as immunoregulators of pregnancy. Reprod. Biol. Endocrinol., 1, 119 (2003).
T. Nagamatsu and D. J. Schust, The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod. Sci., 17, No. 3, 209-218 (2010).
T. Nagamatsu and D. J. Schust, The contribution of macrophages to normal and pathological pregnancies. Am. J. Reprod. Immunol., 63, No. 6, 460-471 (2010).
A. K. Olsson, A. Dimberg, J. Kreuger, and L. Claesson-Welsh, VEGF receptor signalling – in control of vascular function. Nat. Rev. Mol. Cell Biol., 7, No. 5, 359-371 (2006).
Y. S. Onokhina, T. Y. L’vova, D. Z. Tsitskarava, D. A. Koren’kov, S. A. Sel’kov, and D. I. Sokolov, Effect of factors produced by the placenta on cytokine secretion by THP-1 cells cultured on a 3D scaffold. Bull. Exp. Biol. Med., 156, No. 4, 566-570 (2014).
R. M. Rao, L. Yang, G. Garcia-Cardena, and F. W. Luscinskas, Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res., 101, No. 3, 234-247 (2007).
D. T. Rein, M. Breidenbach, B. Honscheid, U. Friebe-Hoffmann, H. Engel, U. J. Gohring, L. Uekermann, C. M. Kurbacher, and T. Schondorf, Preeclamptic women are deficient of interleukin-10 as assessed by cytokine release of trophoblast cells in vitro. Cytokine, 23, Nos. 4-5, 119-125 (2003).
S. J. Renaud and C. H. Graham, The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol. Invest., 37, No. 5, 535-564 (2008).
D. Ribatti, B. Nico, E. Crivellato, and A. Vacca, Macrophages and tumor angiogenesis. Leukemia, 21, No. 10, 2085-2089 (2007).
T. Schmidt and P. Carmeliet, Blood-vessel formation: Bridges that guide and unite. Nature, 465, 697-699 (2010).
D. Schonkeren, M. L. van der Hoorn, P. Khedoe, G. Swings, E. van Beelen, F. Claas, C. van Kooten, E. de Heer, and S. Scherjon, Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am. J. Pathol., 178, No. 2, 709-717 (2011).
Y. Seval, E. T. Korgun, and R. Demir, Hofbauer cells in early human placenta: possible implications in vasculogenesis and angiogenesis. Placenta, 28, Nos. 8-9, 841-845 (2007).
S. D. Smith, C. E. Dunk, J. D. Aplin, L. K. Harris, and R. L. Jones, Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol., 174, No. 5, 1959-1971 (2009).
S. L Straszewski-Chavez., V. M. Abrahams, and G. Mor, The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr. Rev., 26, No. 7, 877-897 (2005).
C. Sunderkotter, K. Steinbrink, M. Goebeler, R. Bhardwaj, and C. Sorg Macrophages and angiogenesis. J. Leukoc. Biol., 55, No. 3, 410-422 (1994).
E. Vey, J. H. Zhang, and J. M. Dayer, IFN-gamma and 1,25(OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J. Immunol., 149, No. 6, 2040-2046 (1992).
Y. Wang and S. W. Walsh, TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J. Reprod. Immunol., 32, No. 2, 157-169 (1996).
R. B. Wesley 2nd, X. Meng, D. Godin, and Z. S. Galis, Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler. Thromb. Vasc. Biol., 18, No. 3, 432-440 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 1, pp. 26-32, January, 2016
Rights and permissions
About this article
Cite this article
Lvova, T.Y., Stepanova, O.I., Viazmina, L.P. et al. Effect of Factors Secreted by the Placenta on Phenotype of THP-1 Cells Cultured on a 3D Scaffold. Bull Exp Biol Med 161, 162–167 (2016). https://doi.org/10.1007/s10517-016-3368-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-016-3368-4