Abstract
Acute ammonia toxicity was investigated in four developmental stages of the juvenile ide, Leuciscus idus: 1, 10, 20 and 30 days after the first feeding. Mean (±SD) total length of the larvae was 8.5 ± 0.3, 15.7 ± 0.7, 23.0 ± 2.0 and 29.7 ± 2.0 mm, and standard weight was 1.6 ± 0.3, 9.2 ± 5.5, 94.9 ± 31.0 and 196.0 ± 31.7 mg, respectively. The larvae used for toxicity tests were reared in the experimental, closed recirculating system. Groups of fishes (n from 7 to 10; in respect of fish size) were exposed to the ammonium chloride solutions in 1-L glass units. Water temperature was 25 ± 0.1 °C for both the rearing and the toxicity tests. pH was not adjusted and ranged between 8.4 and 8.7. The ammonium chloride solutions were renewed every 12 h. At the same time, dead larvae were counted and removed, and the pH and temperature measurements were taken. Each acute toxicity test duration was 96 h, and lethal concentration LC1, LC50 and LC99 values were calculated for 24, 48, 72 and 96 h. The susceptibility of the ide larvae to ammonia decreased linearly with age up to 20th day and surprisingly increased during the next 10 days. The LC50 (48 h) values ranged from 0.27 mg L−1 of unionized ammonia nitrogen for 1 day after the first feeding (AFF) larvae to 1.42 mg L−1 at day 20 after first feeding. The LC50 (48 h) for 30 days AFF was as high as 0.67 mg L−1. The critical level of the unionized ammonia nitrogen for ide larvae was suggested as 0.21 mg L−1.
Similar content being viewed by others
Introduction
In contrast to mammals, most of the fish species do not excrete urea. The main waste product of the protein catabolism in fish is ammonia excreted through the gills (Wood 1993). Ammonia is a highly toxic chemical compound, and fish experience its toxic levels in both natural environment and intensive culture systems. Toxicity of ammonia depends on the water parameters including pH and temperature, mainly. These two parameters affect the concentration of more toxic form, which is unionized ammonia (UIAN) (Randall and Tsui 2002). UIAN diffuses through the gill membranes easily. Central nervous system is the main target organ for the ammonia intoxication (Randall and Ip 2006; Svobodova et al. 2007). Low ammonia concentration in the water can stimulate fish growth (Wood 2004); however, chronic exposure to ammonia reduces survival and growth in the fish at the early stages of development (Brinkman 2009).
Adhibition of RAS-based rearing at comparatively high temperatures and stocking densities, and application of the high-protein feeds allow for intensification of the fish larvae and juvenile production (van Rijn 1996; Remen et al. 2008). On the other hand, RAS generates high risk of the waste nitrogen compound accumulation. According to Biswas et al. (2006), ambient ammonia and nitrite concentration is a key factor for the optimization of the stocking density as even small concentration of these compounds decreases fish growth, health and survival rate.
Ide Leuciscus idus (L.) is an Eurasian native fish species. In the recent years, a serious decline of the ide populations has been observed in the Central Europe (Hamáčková et al. 2007, Kucharczyk et al. 2008, Aleksejevs and Birzaks 2011). This triggered the need for the production of the ide for the restocking purposes. Golden ide Leuciscus idus melanotus are often kept as ornamental fish and furthermore are used as test fish for toxicological monitoring. According to Krejszeff et al. (2009), ide contains 69–91 % of rheophilic cyprinid production in Poland.
It was recently reported that rearing of the cyprinids larvae may be conducted in very high stocking densities, up to 600 ind. L−1, without the negative effect on their survival (Kupren et al. 2011; Żarski et al. 2011). However, application of such intensive procedures together with the intensive feeding may result in increased level of the ammonia excretion. There is still very little information regarding the effect of the increased ammonia level on freshwater fish larvae. Recent studies have shown that susceptibility of the chub, Leuciscus cephalus (L.), to ammonia decreased during the larval development and the larvae at the onset of the exogenous feeding are extremely sensitive to the ammonia (Gomułka et al. 2011). There are no data on the susceptibility of the ide larvae at different ontogenetic stages to ammonia.
The aim of this study was to determine the acute toxicity of ammonia to ide at four different stages of larval development.
Materials and methods
Broodstock and hatched larvae management
Ide larvae were obtained in the course of the controlled reproduction of the wild spawners caught in the Mosąg Lake (northeast Poland) at the beginning of the reproductive season. The reproduction was performed according to the method described by Krejszeff et al. (2009) with the application of the single hormonal treatment with Ovopel (40 μg of mammalian analogue of gonadoliberin and 20 mg of dopamine antagonist—metoclopramide). The eggs were incubated in Weiss jars at 14 °C. Hatched larvae were transferred to the rearing fiberglass tank (150 L) where larvae were kept till the inflation of the swim bladder at the temperature of 20 °C. Next, larvae were acclimated to the temperature of 25 °C (for 1 day) and transferred to the experimental rearing units.
Rearing procedure
Fifteen 1-L glass rearing units placed in a small reticulating system (described in detail by Krejszeff et al. 2008) were used at the experimental rearing starting from the onset of the exogenous feeding of larvae. That moment of the onset of the exogenous feeding was determined according to the procedure described by Gomulka et al. (2011).
The experimental rearing lasted 30 days. The stocking density for the first 20 days was 150 ind. L−1. Larvae were then randomly restocked for the further rearing at 50 ind. L−1 in the rearing unit. The temperature (25 °C ± 0.1) and photoperiod (12L:12D) were constant throughout the experiment. Fish were fed ad libitum three times daily with the hatched Artemia nauplii. Tanks were cleaned twice daily. Dead larvae were counted and removed. Every 3 days, oxygen saturation (HI 91410, Hanna Instruments, Italy) and the concentrations of ammonia (HI 83214, Hanna Instruments, Italy) and nitrites (using an LF205 photometer, Slandi, Poland) were monitored.
Before each toxicity test, 30 larvae were randomly chosen, euthanatized in a solution of 2-phenoxyethanol (0.4 mL L−1) and photographed under a stereoscopic microscope (Leica MZ 12.5, Germany). These photographs were then used for larvae measurements (±0.01 mm, with ProgRes_ Capture Pro 2.5 software, Jenoptik, Germany). The wet body weight (WBW) of the larvae was determined (±0.1 mg).
Toxicity testing
Test procedure followed the OECD Guideline No. 203 “Fish acute toxicity test.” The first experimental group (D1) was sampled the day when first feeding began (day 1). Food was supplied to all rearing tanks at the day. Before second feeding, 100 randomly chosen (from three tanks) specimens were removed to a separate tank and were starved for the next 24 h. These larvae were then used for the first toxicity test. The next three groups of larvae representing group D10, D20 and D30 were sampled on days 10, 20 and 30 after first feeding (AFF), respectively.
Next, fish were randomly divided into nine experimental groups subjected to a set of increasing ammonia concentrations. Larvae (n = 10 for the D1, D10 and D20 tests and n = 7 for the D30 test) were then randomly allocated in 1-L test glass units placed in a water bath. The test water temperature was 25 ± 0.1 °C. Each experiment was conducted in duplicate. Each replicate was provided with the control group. Each unit was gently aerated. Ammonia solutions were renewed every 24 h. The test duration was 96 h. The water temperature, pH and total ammonia nitrogen (TAN) concentration were measured twice a day. The TAN concentration was measured with a multiparametric analyzer (HI 83214, Hanna Instruments, Italy). The number of dead fish was recorded, and dead fish were removed twice a day.
Ammonia solutions
A new stock solution of 10 g L−1 of ammonium chloride (NH4Cl pure p.a.; Chempur, Poland) in the redistilled water was prepared every day. Defined volumes of the stock solution were added to the clean experimental units according to the established set of concentrations. Each vessel was then filled with laboratory tap water up to 1 L. The tap water was aerated and heated to 25 ± 0.1 °C for 24 h before use. The tap water parameters were as follows (mean ± SD): hardness 274 ± 15 ppm CaCO3 and conductivity 548 ± 24 μS cm−1. No pH adjustment was applied.
Calculations and data analysis
The mean temperature and mean pH were calculated for each test unit and for each exposure time point (12, 24, 36, 48, 60, 72, 84 and 96 h). Then, mean UIAN concentration was calculated according to the algorithm given in “Update of Ambient Water Quality Criteria for Ammonia” (USA Environmental Protection Agency publication number EPA-822-R-99-014; 1999). The concentrations of UIAN that kills 1, 5, 50, 95 and 99 % of exposed fish (LC1, LC5, LC50, LC95 and LC99, respectively) were calculated with Probit software (EPA, version 1.5) for each exposure time point, if possible. The relationships between measured parameters were analyzed using the nonlinear regression method. The significance level of 0.05 was used.
Results
Controlled rearing
Throughout the rearing period, a very low mortality rate was observed, which did not exceed 4 % in any of the rearing units at day 30. At the first, tenth, twentieth and thirtieth days of the experiment, the length of the larvae and the wet body weight were 8.5 ± 0.3, 15.7 ± 0.7, 23.0 ± 2.0 and 29.7 ± 2.0 mm and 1.6 ± 0.3, 9.2 ± 5.5, 94.9 ± 31.0 and 196.0 ± 31.7 mg, respectively. The correlation between age and size was almost full (r 2 = 0.998 for age and length, p < 0.05). Throughout the rearing period, the concentration of ammonia and nitrites did not exceed 0.05 and 0.05 mg L−1, respectively. The oxygen level in rearing tanks did not drop below 80 % saturation during 30 days of rearing.
Acute ammonia toxicity
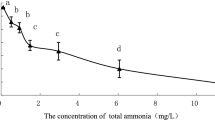
The susceptibility of the ide larvae to ammonia decreased linearly with age till 20th day after the first feeding and then surprisingly increased within next 10 days (Table 1). The high positive correlation was found between fish weight and LC50 values (R 2 = 0.9446, p < 0.05) (Fig. 1a) for the first 20 days AFF. The correlation between fish length and LC50 values was almost full (R 2 = 0.9996, p < 0.05) for the same period (Fig. 1b).
The LC50 (48 h) values ranged from 0.27 mg L−1 of UIAN for D1 larvae to 1.42 mg L−1 for D20 larvae (Table 1). The LC50 (48 h) for D30 was estimated at 0.67 mg L−1. The last value was calculated from the equation of the linear regression: y = −0.003x + 0.82, where y is the LC50 value and x is the time of the exposure (the Pearson’s correlation coefficient r = 0.93, p < 0.05).
Discussion
The relationship between fish age and susceptibility to ammonia toxicity has been studied in various fish species. It is not clear and different results are reported by numerous authors (see Gomułka et al. 2011).
Following Vedel et al. (1998) and Wicks and Randall (2002), Gomułka et al. (2011) hypothesized that increasing resistance to ammonia results from a general increment in both physiological efficiency and muscle capability for glutamine storage in larval chub. However, in case of the ide, the correlation between age and size was almost full during 30 days of the experiment and thus other factor was responsible for the drop of ide resistance to ammonia. Larval development of ide and chub is similar; however, the time for reaching particular developmental stage is species specific and dependent on the environmental conditions (Kupren et al. 2011). We can only speculate that some developmental changes (e.g., rapidly increasing gill surface) enabled a rise of ammonia inside the fish body and, at the same time, the ability to ammonia removal or temporal storage did not increase as quickly as changes enabling the rise of blood ammonia level. Further studies on these phenomena are needed.
Ide is relatively susceptible to UIAN during its larval development when compared to other fish species (for details, see the review of US Environmental Protection Agency publication “1999 Update of Ambient Water Quality Criteria for Ammonia” EPA-822-R-99-014).
The drop of the resistance to ammonia at the end of larval development may result in mortality during transport or other manipulations involving stress and high stocking densities. In Poland, 5-month-old fry are usually used for stocking purposes. Older fish seems to be more resistant to ammonia. Hendriks and Stouten (1993) reported ammonia LC50 for 24 h for adult ide as high as 28.0 mg L−1.
Conclusions
We can assume that larval ide is particularly susceptible to ammonia toxicity at the beginning of larval development. Thus, during that period, special attention should be paid to ammonia concentration monitoring. We suggest using the LC5 for 96 h (0.01 UIAN mg L−1) for the 1st day AFF as a warning level and the LC1 for 24 h (0.21 UIAN mg L−1) (Table 1) as a critical unionized ammonia nitrogen concentration for ide larvae. Larval care procedures should provide efficient ammonia removal or freshwater supply enough to maintain ammonia level below 0.01 UIAN mg L−1 during the first 10 days AFF at least.
Any procedures involving stress or high stocking densities should be avoided during larval development.
Abbreviations
- AFF:
-
After the first feeding
- TAN:
-
Total ammonia nitrogen
- UIAN:
-
Unionized ammonia nitrogen
- RAS:
-
Recirculated aquaculture system
References
Aleksejevs E, Birzaks J (2011) Long-term changes in the Ichthyofauna of Latvia’s inland waters. Sci J Riga Tech Univ 7:9–18
Biswas JK, Sarkar D, Chakraborty P, Bhakta JN, Jana BB (2006) Density dependent ambient ammonium as the key factor for optimization of stocking density of common carp in small holding tanks. Aquaculture 261:952–959
Brinkman SF (2009) Chronic toxicity of ammonia to early life stage rainbow trout. Trans Am Fish Soc 138:433–440
Gomułka P, Żarski D, Kucharczyk D, Kupren K, Krejszeff S, Targońska K (2011) Acute ammonia toxicity during early ontogeny of chub, Leuciscus cephalus (Cyprinidae) Aquat. Living Resour 24:211–217
Hamáčková J, Lepičová A, Prokeš M, Lepič P, Kozák P, Policar T, Stanny LA (2007) Success of nursing ide (Leuciscus idus, L.) fry related to the period of feeding with live food. Aquacult Int 15:255–265
Hendriks AJ, Stouten MDA (1993) Monitoring the response of microcontaminants by dynamic Daphnia magna and Leuciscus idus assays in the Rhine Delta: biological early warning as a useful supplement. Ecotoxicol Environ Saf 26:265–279
Krejszeff S, Żarski D, Kucharczyk D, Kupren K, Targońska K, Mamcarz A (2008) An experimental device for eggs incubation and fish larvae rearing under laboratory conditions. Pol J Nat Sci 25:190–199
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2009) Domestication affects spawning of the ide (Leuciscus idus)-preliminary study. Aquaculture 295:145–147
Kucharczyk D, Targońska K, Żarski D, Kujawa R, Mamcarz A (2008) A review of the reproduction biotechnology for fish from the genus Leuciscus. Arch Pol Fish 16:319–340
Kupren K, Żarski D, Krejszeff S, Kucharczyk D, Targońska K (2011) Effect of stocking density on growth, survival and development of asp Aspius aspius (L.), ide Leuciscus idus (L.) and chub Leuciscus cephalus (L.) larvae during initial rearing under laboratory conditions. Ital J Anim Sci 10:e:34, 178–e:34, 184
Randall DJ, Ip YK (2006) Ammonia as a respiratory gas in water and air-breathing fishes. Resp Physiol Neurobiol 154:216–225
Randall DJ, Tsui TKN (2002) Ammonia toxicity in Fish. Mar Pollut Bull 45:17–23
Remen M, Imsland AK, Steffanson SO, Jonassen TM, Foss A (2008) Interactive effects of ammonia and oxygen on growth and physiological status of juvenile Atlantic cod (Gadus morhua). Aquaculture 274:292–299
Svobodova Z, Machova J, Kroupova H, Smutna M, Groch L (2007) Ammonia autointoxication of common carp, case studies. Aquacult Int 15:277–286
van Rijn J (1996) The potential for integrated biological treatment systems in recirculating fish culture. A review. Aquaculture 139:181–201
Vedel NE, Korsgaard B, Jensen FB (1998) Isolated and combined exposure to ammonia and nitrite in rainbow trout (Oncorhynchus mykiss): effects on electrolyte status, blood respiratory properties and brain glutamine/glutamate concentrations. Aquat Toxicol 41:325–342
Wicks BJ, Randall DJ (2002) The effect of sub-lethal ammonia exposure on fed and unfed rainbow trout: the role of glutamine in regulation of ammonia. Comp Biochem Physiol A 132:275–285
Wood CM (1993) Ammonia and urea metabolism and excretion. In: Ewans DH (ed) Physiology of fishes. CRC Press, Boca Raton, pp 379–425
Wood CM (2004) Dogmas and controversies in the handling of nitrogenous wastes: is exogenous ammonia a growth stimulant in fish? J Exp Biol 207:2043–2054
Żarski D, Kupren K, Targońska K, Krejszeff S, Furgała-Selezniow G, Kucharczyk D (2011) The effect of initial larval stocking density on growth and survival in common barbel Barbus barbus (L.). J Appl Ichthyol 27:1155–1158
Acknowledgments
This study was supported by the project “Innovations in finfish aquaculture with special references to reproduction” (InnovaFish), Operational Programme “Sustainable Development of the Fisheries Sector and Coastal Fishing Areas 2007-2013” (OR14-61724-OR1400003/09/10/11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gomułka, P., Żarski, D., Kupren, K. et al. Acute ammonia toxicity during early ontogeny of ide Leuciscus idus (Cyprinidae). Aquacult Int 22, 225–233 (2014). https://doi.org/10.1007/s10499-013-9677-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9677-y