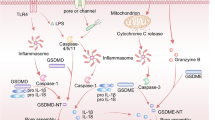
Abstract
Oral squamous cell carcinoma (OSCC) is a malignant tumor with high mortality and poor prognosis. Many OSCC patients have low response rate to current treatments including immunotherapies largely due to the immune-suppressive tumor microenvironment (TME). Chemotherapy could induce immunogenic cell death (ICD), a type of cell death such as pyroptosis and necroptosis, which has proved to be capable to alter the immune-suppressive TME and beneficial for better anti-tumor effect. GSDME, a key protein of pyroptosis, is however often silenced in tumors due to abnormal methylation. To overcome these limitations, we utilizied methyltransferase inhibitor (decitabine, DAC) to trigger pyroptosis of tumor cells, combined with chemodrug cisplatin (DDP) and immune checkpoints inhibitors to amplify the immunotherapies outcomes. To the best of our knowledge, this is the first study of tumor suppressive effect of GSDME in OSCC. Our investigation demonstrated that stimulation of GSDME expression could improve the sensitivity of chemotherapeutics, activate inflammatory tumor cell pyroptosis and alter the tumor immune-suppressive microenvironment, providing an important perspective for clinical OSCC treatment.





Similar content being viewed by others
Data Availability
The data sets used and analyzed in the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article and its supplementary files.
Abbreviations
- OSCC:
-
Oral squamous cell carcinoma
- TME:
-
Tumor microenvironment
- ICD:
-
Immunogenic cell death
- DAC:
-
Decitabine
- DDP:
-
Cisplatin
- HNSCC:
-
Head and neck squamous cell carcinoma
- GSDME:
-
Gasdermin E
References
Chow LQM (2020) Head and neck cancer. N Engl J Med 382(1):60–72
Johnson D, Burtness B, Leemans C, Lui V, Bauman J, Grandis J (2020) Head and neck squamous cell carcinoma. Nat Rev 6(1):92
Vermorken J, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Eng J Med 359(11):1116–1127
Binnewies M, Roberts E, Kersten K et al (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24(5):541–550
Duan Q, Zhang H, Zheng J, Zhang L (2020) Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 6(7):605–618
Peltanova B, Raudenska M, Masarik M (2019) Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mole Cancer 18(1):63
Saddawi-Konefka R, Simon A, Sumner W, Sharabi A, Mell L, Cohen E (2021) Defining the role of immunotherapy in the curative treatment of locoregionally advanced head and neck cancer: promises, challenges, and opportunities. Frontiers Oncol 11:738626
Mei Z, Huang J, Qiao B, Lam AK (2020) Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int J Oral Sci 12(1):16
Dai X, Wang D, Zhang J (2021) Programmed cell death, redox imbalance, and cancer therapeutics. Apoptosis 26:385–414
Zhang Y, Chen X, Gueydan C, Han J (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28(1):9–21
Van Laer L, Huizing E, Verstreken M et al (1998) Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Gene 20(2):194–197
Erkes DA, Cai W, Sanchez IM et al (2020) Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov 10(2):254–269
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547(7661):99–103
Wang S, Zhang MJ, Wu ZZ et al (2022) GSDME is related to prognosis and response to chemotherapy in oral cancer. J Dental Res 101(7):848–858
Zhang Z, Zhang Y, Xia S et al (2020) Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579(7799):415–420
Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T (2000) Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamma Geno 11(9):718–724
Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, Wang K, Sun X, Zheng J (2019) Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Dea Dise 10(3):193
Adelstein D, Gillison M, Pfister D et al (2017) NCCN guidelines insights: head and neck cancers, version 2.2017. J Nat Compre Cancer Net 15(6):761–70
Gal-Yam E, Saito Y, Egger G, Jones P (2008) Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med 59:267–80
Akino K, Toyota M, Suzuki H et al (2007) Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer Sci 98(1):88–95
Kim M, Chang X, Yamashita K et al (2008) Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene 27(25):3624–3634
Wijermans P, Lübbert M, Verhoef G, Bosly A, Ravoet C, Andre M, Ferrant A (2000) Low-dose 5-aza-2’-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol 18(5):956–962
Silverman L, Demakos E, Peterson B et al (2002) Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20(10):2429–2440
Pardoll D (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev 12(4):252–264
Wise-Draper T, Gulati S, Palackdharry S et al (2022) Phase II clinical trial of neoadjuvant and adjuvant pembrolizumab in resectable local-regionally advanced head and neck squamous cell carcinoma. Clin Cancer Res 28(7):1345–1352
Saada-Bouzid E, Peyrade F, Guigay J (2019) Immunotherapy in recurrent and or metastatic squamous cell carcinoma of the head and neck. Curr Opin Oncol 31(3):146–151
Ferris R, Blumenschein G, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. New Engl J Med 375(19):1856–1867
Ibrahim J, Op de Beeck K, Fransen E, Croes L, Beyens M, Suls A, Vanden Berghe W, Peeters M, Van Camp G (2019) Methylation analysis of Gasdermin E shows great promise as a biomarker for colorectal cancer. Cancer Med 8(5):2133–2145
Souza CF, Sabedot TS, Malta TM, Stetson L, Morozova O, Sokolov A et al (2018) A distinct DNA methylation shift in a subset of glioma CpG island methylator phenotypes during tumor recurrence. Cell Rep 23(2):637–651
Wallach D, Kang T, Kovalenko A (2014) Concepts of tissue injury and cell death in inflammation: a historical perspective. Nature Rev Immunol 14(1):51–59
Nowak-Sliwinska P, Griffioen AW (2018) Apoptosis on the move. Apoptosis 23(5–6):251–254
Wallach D, Kang TB, Dillon CP, Green DR (2016) Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352(6281):aaf2154
Kesavardhana S, Malireddi RKS, Kanneganti TD (2020) Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol 38:567–95
Lu H, Zhang S, Wu J, Chen M, Cai MC (2018) Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clin Cancer Res 24(23):6066–6077
Philip M, Rowley DA, Schreiber H (2004) Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol 14(6):433–439
Vakkila J, Lotze MT (2004) Inflammation and necrosis promote tumour growth. Nature Rev Immunol 4(8):641–648
Ma S, Song W, Xu Y, Si X, Zhang Y, Tang Z, Chen X (2020) A ROS-responsive aspirin polymeric prodrug for modulation of tumor microenvironment and cancer immunotherapy. CCS Chem 2(6):390–400
Acknowledgements
The authors thank the National Center for Protein Science at Peking University in Beijing, China, for assistance with Nikon A1R confocal microscopy photography and instruction of using flow cytometer. We thank Su Lab’s help in the cell experiments at Biomedical Pioneering Innovation Center, State Key Laboratory of Protein and Plant Gene Research, School of Life Science, Peking University. We thank for Figdraw website for the picture drawing.
Funding
This work was supported by Discipline Development Fund of School of Stomatology, Peking University.
Author information
Authors and Affiliations
Contributions
ZM: contributed to conceptualization, methodology, data analysis, writing original draft, writing, review and editing; XYC: contributed to conceptualization, methodology, data analysis; YC: contributed to data analysis; XDS: contributed to original draft review; SXL: contributed to conceptualization, funding acquisition, supervision, writing-review and editing; SCW: contributed to supervision, project administration, writing-review and editing. All authors gave final approval and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
This study was performed in line with the principles of the Medical Ethics Committee and Biosafety Management Committee of Peking University (Approval Number LA2019197).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zi, M., Xingyu, C., Yang, C. et al. Improved antitumor immunity of chemotherapy in OSCC treatment by Gasdermin-E mediated pyroptosis. Apoptosis 28, 348–361 (2023). https://doi.org/10.1007/s10495-022-01792-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01792-3