Abstract
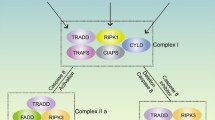
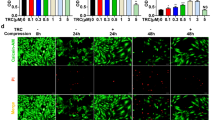
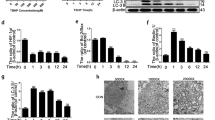
The aim of this study was to investigate whether RIPK1 mediated mitochondrial dysfunction and oxidative stress contributed to compression-induced nucleus pulposus (NP) cells necroptosis and apoptosis, together with the interplay relationship between necroptosis and apoptosis in vitro. Rat NP cells underwent various periods of 1.0 MPa compression. To determine whether compression affected mitochondrial function, we evaluated the mitochondrial membrane potential, mitochondrial permeability transition pore (mPTP), mitochondrial ultrastructure and ATP content. Oxidative stress-related indicators reactive oxygen species, superoxide dismutase and malondialdehyde were also assessed. To verify the relevance between oxidative stress and necroptosis together with apoptosis, RIPK1 inhibitor necrostatin-1(Nec-1), mPTP inhibitor cyclosporine A (CsA), antioxidants and small interfering RNA technology were utilized. The results established that compression elicited a time-dependent mitochondrial dysfunction and elevated oxidative stress. Nec-1 and CsA restored mitochondrial function and reduced oxidative stress, which corresponded to decreased necroptosis and apoptosis. CsA down-regulated mitochondrial cyclophilin D expression, but had little effects on RIPK1 expression and pRIPK1 activation. Additionally, we found that Nec-1 largely blocked apoptosis; whereas, the apoptosis inhibitor Z-VAD-FMK increased RIPK1 expression and pRIPK1 activation, and coordinated regulation of necroptosis and apoptosis enabled NP cells survival more efficiently. In contrast to Nec-1, SiRIPK1 exacerbated mitochondrial dysfunction and oxidative stress. In summary, RIPK1-mediated mitochondrial dysfunction and oxidative stress play a crucial role in NP cells necroptosis and apoptosis during compression injury. The synergistic regulation of necroptosis and apoptosis may exert more beneficial effects on NP cells survival, and ultimately delaying or even retarding intervertebral disc degeneration.







Similar content being viewed by others
Abbreviations
- IVD:
-
Intervertebral disc
- IVDD:
-
IVD degeneration
- NP:
-
Nucleus pulposus
- RIPK1:
-
Receptor-interacting protein kinase 1
- RIPK3:
-
Receptor-interacting protein kinase 3
- MLKL:
-
Mixed lineage kinase domain-like
- PCD:
-
Programmed cell death
- NF-κB:
-
Nuclear factor-κB
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- mtROS:
-
Mitochondrial ROS
- LDH:
-
Lactate dehydrogenase
- CypD:
-
Cyclophilin D
- MMP:
-
Mitochondrial membrane potential
- mPTP:
-
Mitochondrial permeability transition pore
- SiRNA:
-
Small interfering RNA
- Nec-1:
-
Necrostatin-1
- z-VAD:
-
Z-VAD-FMK
- CsA:
-
Cyclosporine A
- NAC:
-
N-acetyl-l-cysteine
- BHA:
-
Butyl-4-hydroxyanisole
- NS:
-
No significant statistical significance
References
Yurube T, Hirata H, Kakutani K, Maeno K, Takada T, Zhang Z, Takayama K, Matsushita T, Kuroda R, Kurosaka M, Nishida K (2014) Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther 16:R31
Yurube T, Takada T, Suzuki T, Kakutani K, Maeno K, Doita M, Kurosaka M, Nishida K (2012) Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther 14:R51
Sowa G, Agarwal S (2008) Cyclic tensile stress exerts a protective effect on intervertebral disc cells. Am J Phys Med Rehabil 87:537–544
Wang C, Gonzales S, Levene H, Gu W, Huang CY (2013) Energy metabolism of intervertebral disc under mechanical loading. J Orthop Res 31:1733–1738
Hirata H, Yurube T, Kakutani K, Maeno K, Takada T, Yamamoto J, Kurakawa T, Akisue T, Kuroda R, Kurosaka M, Nishida K (2014) A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J Orthop Res 32:455–463
Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG (2002) Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine J 27:2631–2644
Sudo H, Minami A (2011) Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheumatol 63:1648–1657
Sudo H, Minami A (2010) Regulation of apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2 overexpression. J Orthop Res 28:1608–1613
Ma KG, Shao ZW, Yang SH, Wang J, Wang BC, Xiong LM, Wu Q, Chen SF (2013) Autophagy is activated in compression-induced cell degeneration and is mediated by reactive oxygen species in nucleus pulposus cells exposed to compression. Osteoarthr Cartil 21:2030–2038
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK (2015) Differential roles of RIPK1 and RIPK3 in TNF induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis 6:e1636
Chen S, Lv X, Hu B, Shao Z, Wang B, Ma K, Lin H, Cui M (2017) RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death. Apoptosis 22:626–638
Agarwal S, Yadav A, Tiwari SK, Seth B, Chauhan LK, Khare P, Ray RS, Chaturvedi RK (2016) Dynamin related protein 1 inhibition mitigates bisphenol A-mediated alterations in mitochondrial dynamics and neural stem cell proliferation and differentiation. J Biol Chem 291:15923–15939
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y (2017) Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13:498–507
Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, Kidd G, Wakefield R, Frase S, Krautwald S, Linkermann A, Green DR (2013) Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep 5:878–885
Moriwaki K, Farias Luz N, Balaji S, De Rosa MJ, O’Donnell CL, Gough PJ, Bertin J, Welsh RM, Chan FK (2016) The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol 196:407–415
Liu X, Gao RW, Li M, Si CF, He YP, Wang M, Yang Y, Zheng QY, Wang CY (2016) The ROS derived mitochondrial respiration not from NADPH oxidase plays key role in celastrol against angiotensin II-mediated HepG2 cell proliferation. Apoptosis 21:1315–1326
Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W, Vandenabeele P (2016) Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17:922–930
Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao L, Liu J (2016) Geniposide prevents hypoxia reoxygenation-induced apoptosis in H9c2 cells: improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell Physiol Biochem 39:407–421
Ahsan A, Han G, Pan J, Liu S, Padhiar AA, Chu P, Sun Z, Zhang Z, Sun B, Wu J, Irshad A, Lin Y, Peng J, Tang Z (2015) Phosphocreatine protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Apoptosis 20:1563–1576
Ding F, Shao ZW, Yang SH, Wu Q, Gao F, Xiong LM (2012) Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis 17:579–590
Seya T, Shime H, Takaki H, Azuma M, Oshiumi H, Matsumoto M (2012) TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. Oncoimmunology 1:917–923
Zhao L, Lin H, Chen S, Chen S, Cui M, Shi D, Wang B, Ma K, Shao Z (2017) Hydrogen peroxide induces programmed necrosis in rat nucleus pulposus cells through the RIP1/RIP3-PARP-AIF pathway. J Orthop Res. https://doi.org/10.1002/jor.23751
Wang F, Cai F, Shi R, Wang XH, Wu XT (2016) Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthr Cartil 24:398–408
Jiang LB, Cao L, Yin XF, Yasen M, Yishake M, Dong J, Li XL (2015) Activation of autophagy via Ca2+ dependent AMPK/mTOR pathway in rat notochordal cells is a cellular adaptation under hyperosmotic stress. Cell Cycle 14:867–879
Hunter CJ, Matyas JR, Duncan NA (2003) The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 9:667–677
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90:1193–1209
Carlisi D, Buttitta G, Di Fiore R, Scerri C, Drago-Ferrante R, Vento R, Tesoriere G (2016) Parthenolide and DMAPT exert cytotoxic effects on breast cancer stem-like cells by inducing oxidative stress, mitochondrial dysfunction and necrosis. Cell Death Dis 7:e2194
Chen Q, Chen X, Han C, Wang Y, Huang T, Du Y, Dong Z (2016) FGF-2 transcriptionally down-regulates the expression of BNIP3L via PI3K/Akt/FoxO3a signaling and inhibits necrosis and mitochondrial dysfunction induced by high concentrations of hydrogen peroxide in H9c2 cells. Cell Physiol Biochem 40:1678–1691
Xu D, Jin H, Wen J, Chen J, Chen D, Cai N, Wang Y, Wang J, Chen Y, Zhang X, Wang X (2017) Hydrogen sulfide protects against endoplasmic reticulum stress and mitochondrial injury in nucleus pulposus cells and ameliorates intervertebral disc degeneration. Pharmacol Res 117:357–369
Niu CC, Lin SS, Yuan LJ, Chen LH, Wang IC, Tsai TT, Lai PL, Chen WJ (2013) Hyperbaric oxygen treatment suppresses MAPK signaling and mitochondrial apoptotic pathway in degenerated human intervertebral disc cells. J Orthop Res 31:204–209
Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI (2016) Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension 67:1218–1227
Abramov AY, Duchen MR (2008) Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta 1777:953–964
Fakharnia F, Khodagholi F, Dargahi L, Ahmadiani A (2017) Prevention of cyclophilin D-mediated mPTP opening using cyclosporine-A alleviates the elevation of necroptosis, autophagy and apoptosis-related markers following global cerebral ischemia-reperfusion. J Mol Neurosci 61:52–60
Li S, Guo J, Ying Z, Chen S, Yang L, Chen K, Long Q, Qin D, Pei D, Liu X (2016) Valproic acid-induced hepatotoxicity in Alpers syndrome is associated with mitochondrial permeability transition pore opening-dependent apoptotic sensitivity in an induced pluripotent stem cell model. Hepatology 61:1730–1739
Gharanei M, Hussain A, Janneh O, Maddock HL (2013) Doxorubicin induced myocardial injury is exacerbated following ischaemic stress via opening of the mitochondrial permeability transition pore. Toxicol Appl Pharmacol 268:149–156
Ye YC, Wang HJ, Yu L, Tashiro S, Onodera S, Ikejima T (2012) RIP1-mediated mitochondrial dysfunction and ROS production contributed to tumor necrosis factor alpha-induced L929 cell necroptosis and autophagy. Int Immunopharmacol 14:674–682
Kim SY, Shim MS, Kim KY, Weinreb RN, Wheeler LA, Ju WK (2014) Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis 5:e1105
Zhu XD, Chi JY, Liang HH, Huangfu LT, Guo ZD, Zou H, Yin XH (2016) MicroRNA-377 mediates cardiomyocyte apoptosis induced by cyclosporin A. Can J Cardiol 32:1249–1259
Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, Mookhtiar AK, Zhao H, Xu D, Shan B, Najafov A, Gao G, Akira S, Yuan J (2017) Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun 8:359
Christofferson. Dana E, Yuan. Junying (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22:263–268
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P (1998) The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297–303
Obitsu S, Sakata K, Teshima R, Kondo K (2013) Eleostearic acid induces RIP1-mediated atypical apoptosis in a kinase-independent manner via ERK phosphorylation, ROS generation and mitochondrial dysfunction. Cell Death Dis 4:e674
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 81572203), the National Key Research and Development Program of China (Grant No. 2016YFC1100100) and the Youth Innovation Fund of The First Affiliated Hospital of Zhengzhou University (Grant No. YNQN 2017037).
Author information
Authors and Affiliations
Contributions
SC, XL, BH and ZS designed the research. SC, XL, BH and LZ performed the experiments. SC, XL, and SL acquired and analyzed the data. SC, XL, BH, XQ, HL and JX contributed to writing of the manuscript. Finally, all authors have reviewed and approved the final submitted manuscript. The integrity of this work is guaranteed by Dr Songfeng Chen, Dr Xiao Lv, Dr Binwu Hu, and Dr Zengwu Shao.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, S., Lv, X., Hu, B. et al. Critical contribution of RIPK1 mediated mitochondrial dysfunction and oxidative stress to compression-induced rat nucleus pulposus cells necroptosis and apoptosis. Apoptosis 23, 299–313 (2018). https://doi.org/10.1007/s10495-018-1455-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1455-x