Abstract
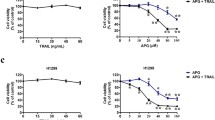
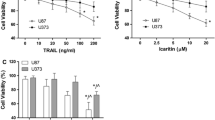
TRAIL, an apoptosis inducing cytokine currently in phase II clinical trial, was investigated for its capability to induce apoptosis in six different human tumor cell lines out of which three cell lines showed resistance to TRAIL induced apoptosis. To investigate whether Anacardic acid (A1) an active component of Anacardium occidentale can sensitize the resistant cell lines to TRAIL induced apoptosis, we treated the resistant cells with suboptimal concentration of A1 and showed that it is a potent enhancer of TRAIL induced apoptosis which up-regulates the expression of both DR4 and DR5 receptors, which has been observed in the cellular, protein and mRNA levels. The death receptors upregulation consequent to A1 treatment was corroborated by the activation of p53 as well as phosphorylation of p38 and JNK MAP kinases and concomitant inactivation of NFκβ and ERK signaling cascades. Also, A1 modulated the expression of key apoptotic players like Bax, Bcl-2 and CAD along with the abatement of tumor angiogenesis in vivo in EAT mouse model. Thus, post A1 treatment the TRAIL resistant cells turned into TRAIL sensitive cells. Hence our results demonstrate that A1 can synergize TRAIL induced apoptosis through the upregulation of death receptors and downregulation of anti-apoptotic proteins in cancer context.







Similar content being viewed by others
Abbreviations
- A1:
-
Anacardic acid
- TRAIL:
-
TNFα related apoptosis inducing ligand
- DRs:
-
Death receptors
- DR4, DR5:
-
Death receptor 4 and 5
- DcR1, DcR2:
-
Decoy receptor 1 and 2
- FasL:
-
FAS ligand
- TNFα:
-
Tumor necrosis factor α
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B-cell lymphoma 2 protein
- Cad:
-
Caspase activated DNase
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer. GLOBOCAN 2008
Bonavida B, Khineche S, Huerta-Yepez S, Garbán H (2006) Therapeutic potential of nitric oxide in cancer. Drug Resist Updates 9(3):157–173
Almasan A, Ashkenazi A (2003) Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev 14(3):337–348
Ashkenazi A, Pai R, Fong S, Leung S, Lawrence D, Marsters S et al (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig 104(2):155
Zhang L, Fang B (2005) Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12(3):228–237
Arizono Y, Yoshikawa H, Naganuma H, Hamada Y, Nakajima Y, Tasaka K (2003) A mechanism of resistance to TRAIL/Apo2L-induced apoptosis of newly established glioma cell line and sensitisation to TRAIL by genotoxic agents. Br J Cancer 88(2):298–306
Kimberley FC, Screaton GR (2004) Following a TRAIL: update on a ligand and its five receptors. Cell Res 14(5):359–372
Griffith TS, Lynch DH (1998) TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol 10(5):559–563
Thome M, Schneider P, Hofmann K, Fickenscher H, Meini E, Neipel F et al (1997) Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386(6624):517–521
Dan’ura T, Kawai A, Morimoto Y, Naito N, Yoshida A, Inoue H (2002) Apoptosis and expression of its regulatory proteins in soft tissue sarcomas. Cancer Lett 178(2):167–174
Jung E, Lim J, Lee T, Park J, Choi K, Kwon T (2005) Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis 26(11):1905–1913
Shenoy K, Wu Y, Pervaiz S (2009) LY303511 enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via hydrogen peroxide–mediated mitogen-activated protein kinase activation and up-regulation of death receptors. Cancer Res 69(5):1941–1950
Prasad S, Yadav V, Kannappan R, Aggarwal B (2011) Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent Up-regulation of death receptors evidence for the role of reactive oxygen species and JNK. J Biol Chem 286(7):5546–5557
Maksimovic-Ivanic D, Stosic-Grujicic S, Nicoletti F, Mijatovic S (2012) Resistance to TRAIL and how to surmount it. Immunol Res 52(1–2):157–168
Tsai WS, Yeow WS, Chua A, Reddy RM, Nguyen DM, Schrump DS et al (2006) Enhancement of Apo2L/TRAIL-mediated cytotoxicity in esophageal cancer cells by cisplatin. Mol Cancer Ther 5(12):2977–2990
Kim SH, Ricci MS, El-Deiry WS (2008) Mcl-1: a gateway to TRAIL sensitization. Cancer Res 68(7):2062–2064
Kim M, Liao J, Dowling M, Voong KR, Parker SE, Wang S et al (2008) TRAIL inactivates the mitotic checkpoint and potentiates death induced by microtubule-targeting agents in human cancer cells. Cancer Res 68(9):3440–3449
Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M et al (2008) Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-κB–regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-κBα kinase, leading to potentiation of apoptosis. Blood 111(10):4880–4891
Hemshekhar M, Sebastin SM, Kemparaju K, Girish KS (2012) Emerging roles of anacardic acid and its derivatives: a pharmacological overview. Basic Clin Pharmacol Toxicol 110(2):122–132
Tan J, Chen B, He L, Tang Y, Jiang Z, Yin G et al (2012) Anacardic acid (6-pentadecylsalicylic acid) induces apoptosis of prostate cancer cells through inhibition of androgen receptor and activation of p53 signaling. Chin J Cancer Res 24(4):275–283
Shilpa P, Kaveri K, Salimath BP (2015) Anti-metastatic action of anacardic acid targets VEGF-induced signalling pathways in epithelial to mesenchymal transition. Drug Discov Ther 9(1):53–65
Gantert M, Lewrick F, Adrian JE, Rössler J, Steenpaß T, Schubert R et al (2009) Receptor-specific targeting with liposomes in vitro based on sterol-PEG1300 anchors. Pharm Res 26(3):529–538
Zeng H, Sanyal S, Mukhopadhyay D (2001) Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem 276(35):32714–32719
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277(5327):815–818
Sheridan JP, Marsters SA, Pitt RM, Gurney A, Skubatch M, Baldwin D et al (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277(5327):818–821
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2(4):277–288
Lauricella M, Ciraolo A, Carlisi D, Vento R, Tesoriere G (2012) SAHA/TRAIL combination induces detachment and anoikis of MDA-MB231 and MCF-7 breast cancer cells. Biochimie 94(2):287–299
Kahana S, Finniss S, Cazacu S, Xiang C, Lee H, Brodie S et al (2011) Proteasome inhibitors sensitize glioma cells and glioma stem cells to TRAIL-induced apoptosis by PKCε-dependent downregulation of AKT and XIAP expressions. Cell Signal 23(8):1348–1357
Pellerito O, Calvaruso G, Portanova P, De Blasio A, Santulli A, Vento R et al (2010) The synthetic cannabinoid WIN 55,212-2 sensitizes hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating p8/CCAAT/enhancer binding protein homologous protein (CHOP)/death receptor 5 (DR5) axis. Mol Pharmacol 77(5):854–863
Hamad FB, Mubofu EB (2015) Potential biological applications of bio-based anacardic acids and their derivatives. Int J Mol Sci 16(4):8569–8590
Takeda K, Stagg J, Yagita H, Okumura K, Smyth MJ (2007) Targeting death-inducing receptors in cancer therapy. Oncogene 26(25):3745–3757
Sung B, Ravindran J, Prasad S, Pandey MK, Aggarwal BB (2010) Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J Biol Chem 285(46):35418–35427
Liu X, Yue P, Khuri FR, Sun S (2004) p53 upregulates death receptor 4 expression through an intronic p53 binding site. Cancer Res 64(15):5078–5083
Wu GS, Burns TF, McDonald ER 3rd, Jiang W, Meng R, Krantz ID et al (1997) KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet 17(2):141
Sun S, Yue P, Hong WK, Lotan R (2000) Augmentation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by the synthetic retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) through up-regulation of TRAIL receptors in human lung cancer cells. Cancer Res 60(24):7149–7155
Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS et al (1998) p53-dependent and-independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor α. Cancer Res 58(8):1593–1598
Azijli K, Yuvaraj S, van Roosmalen I, Flach K, Giovannetti E, Peters GJ et al (2013) MAPK p38 and JNK have opposing activities on TRAIL-induced apoptosis activation in NSCLC H460 cells that involves RIP1 and caspase-8 and is mediated by Mcl-1. Apoptosis 18(7):851–860
Cheng H, Hong B, Zhou L, Allen JE, Tai G, Humphreys R et al (2012) Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: Evidence for the role of c-Jun N-terminal kinase activation. Cell Cycle 11(17):3312–3323
Kang C, Moon D, Choi YH, Choi I, Moon S, Kim WJ et al (2011) Piceatannol enhances TRAIL-induced apoptosis in human leukemia THP-1 cells through Sp1-and ERK-dependent DR5 up-regulation. Toxicol In Vitro 25(3):605–612
Lepage C, Léger DY, Bertrand J, Martin F, Beneytout JL, Liagre B (2011) Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett 301(2):193–202
Krishna M, Narang H (2008) The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci 65(22):3525–3544
Fulda S, Wick W, Weller M, Debatin KM (2002) Smac agonists sensitize for Apo2L/TRAIL-or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med 8(8):808–815
Ravi R, Bedi A (2002) Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res 62(15):4180–4185
Kruyt FA (2008) TRAIL and cancer therapy. Cancer Lett 263(1):14–25
Acknowledgments
We acknowledge funding by Department of Biotechnology, New Delhi, India, BT/IN/German/06/BPS/2010 and BMBF-IND 10/026, to BPS and JR respectively, University Grants Commission-Government of India No. f 4-1/2013SAP II and f.No. 14/4/2012(NS/PE). The discussions and help given by Prof. Dr. Regine Süss and Dr. V. Rengaswamy for liposome studies is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2016_1223_MOESM1_ESM.tif
Supplementary material 1 (TIFF 2773 kb) Sup Fig. 1 (a) Liposome size was determined by dynamic light scattering using a zeta potential/particle sizer of freshly prepared liposomes was performed by diluting in isotonic HBS buffer. (b) In vitro cytotoxicity of A1-liposome formulation and free drug A1 (100 µg) on 8 human tumor cell lines: 5x104 cells were seeded in 46 well plates and treated with A1-liposome formulation, A1 alone, free liposomes and untreated cells for 12 h. The cytotoxicity was assessed by trypan blue dye exclusion method
Rights and permissions
About this article
Cite this article
Harsha Raj, M., Yashaswini, B., Rössler, J. et al. Combinatorial treatment with anacardic acid followed by TRAIL augments induction of apoptosis in TRAIL resistant cancer cells by the regulation of p53, MAPK and NFκβ pathways. Apoptosis 21, 578–593 (2016). https://doi.org/10.1007/s10495-016-1223-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1223-8