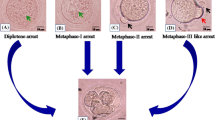
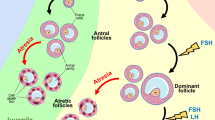
Abstract
The vertebrate ovary is an extremely dynamic organ in which excessive or defective follicles are rapidly and effectively eliminated early in ontogeny and thereafter continuously throughout reproductive life. More than 99% of follicles disappear, primarily due to apoptosis of granulosa cells, and only a minute fraction of the surviving follicles successfully complete the path to ovulation. The balance between signals for cell death and survival determines the destiny of the follicles. An abnormally high rate of cell death followed by atresia can negatively affect fertility and eventually lead irreversibly to premature ovarian failure. In this review we provide a short overview of the role of programmed cell death in prenatal differentiation of the primordial germ cells and in postnatal folliculogenesis. We also discuss the issue of neo-oogenesis. Next, we highlight molecules involved in regulation of granulosa cell apoptosis. We further discuss the potential use of scores for apoptosis in granulosa cells and characteristics of follicular fluid as prognostic markers for predicting the outcome of assisted reproduction. Potential therapeutic strategies for combating premature ovarian failure are also addressed.


Similar content being viewed by others
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501. doi:10.1083/jcb.119.3.493
Coulombre J, Russel E (1954) Analysis of pleiotropism at the W-locus in the mouse: the effect of W and W v substitution upon postnatal development of germ cells. J Exp Zool 126:277–296. doi:10.1002/jez.1401260207
Zuckerman S (1951) The number of oocytes in the mature ovary. Recent Prog Horm Res 6:63–108
Kingery HM (1917) Oogenesis in the white mouse. J Morphol 30:261–316. doi:10.1002/jmor.1050300108
Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL (2004) Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428:145–150. doi:10.1038/nature02316
Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y et al (2005) Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122:303–315. doi:10.1016/j.cell.2005.06.031
Molyneaux K, Wylie C (2004) Primordial germ cell migration. Int J Dev Biol 48:537–544. doi:10.1387/ijdb.041833km
Elvin JA, Matzuk MM (1998) Mouse models of ovarian failure. Rev Reprod 3:183–195. doi:10.1530/ror.0.0030183
Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA (2006) Ovarian follicle development and transgenic mouse models. Hum Reprod Update 12:537–555. doi:10.1093/humupd/dml022
Primig M, Wiederkehr C, Basavaraj R, Sarrauste de Menthiere C, Hermida L, Koch R et al (2003) GermOnline, a new cross-species community annotation database on germ-line development and gametogenesis. Nat Genet 35:291–292. doi:10.1038/ng1203-291
Leo CP, Vitt UA, Hsueh AJ (2000) The Ovarian Kaleidoscope database: an online resource for the ovarian research community. Endocrinology 141:3052–3054. doi:10.1210/en.141.9.3052
MacLaughlin DT, Donahoe PK (2004) Sex determination and differentiation. N Engl J Med 350:367–378. doi:10.1056/NEJMra022784
De Pol A, Marzona L, Vaccina F, Negro R, Sena P, Forabosco A (1998) Apoptosis in different stages of human oogenesis. Anticancer Res 18:3457–3461
Reynaud K, Driancourt MA (2000) Oocyte attrition. Mol Cell Endocrinol 163:101–108. doi:10.1016/S0303-7207(99)00246-4
Baker TG (1963) A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 158:417–433. doi:10.1098/rspb.1963.0055
Gondos B (1978) Oogonia and oocytes in mammals. In: Jones RE (ed) The vertebrate ovary. Plenum Press, New York, pp 83–120
Bowles J, Koopman P (2007) Retinoic acid, meiosis and germ cell fate in mammals. Development 134:3401–3411. doi:10.1242/dev.001107
Hunt PA, Hassold TJ (2008) Human female meiosis: what makes a good egg go bad? Trends Genet 24:86–93. doi:10.1016/j.tig.2007.11.010
Wartenberg H (1982) Development of the early human ovary and role of the mesonephros in the differentiation of the cortex. Anat Embryol (Berl) 165:253–280. doi:10.1007/BF00305481
De Felici M, Klinger FG, Farini D, Scaldaferri ML, Iona S, Lobascio M (2005) Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod Biomed Online 10:182–191
Marcinkiewicz JL, Krishna A, Cheung CM, Terranova PF (1994) Oocytic tumor necrosis factor alpha: localization in the neonatal ovary and throughout follicular development in the adult rat. Biol Reprod 50:1251–1260. doi:10.1095/biolreprod50.6.1251
Tilly JL (2001) Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol 2:838–848. doi:10.1038/35099086
Baum JS, St George JP, McCall K (2005) Programmed cell death in the germline. Semin Cell Dev Biol 16:245–259. doi:10.1016/j.semcdb.2004.12.008
Driancourt MA, Reynaud K, Cortvrindt R, Smitz J (2000) Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod 5:143–152. doi:10.1530/ror.0.0050143
Pesce M, Siracusa G, Giustiniani Q, De Felici M (1994) Histotypic in vitro reorganization of dissociated cells from mouse fetal gonads. Differentiation 56:137–142. doi:10.1046/j.1432-0436.1994.5630137.x
Felici MD, Carlo AD, Pesce M, Iona S, Farrace MG, Piacentini M (1999) Bcl-2 and Bax regulation of apoptosis in germ cells during prenatal oogenesis in the mouse embryo. Cell Death Differ 6:908–915. doi:10.1038/sj.cdd.4400561
Moniruzzaman M, Sakamaki K, Akazawa Y, Miyano T (2007) Oocyte growth and follicular development in KIT-deficient Fas-knockout mice. Reproduction 133:117–125. doi:10.1530/REP-06-0161
Krakauer DC, Mira A (1999) Mitochondria and germ-cell death. Nature 400:125–126. doi:10.1038/22026
Modi DN, Sane S, Bhartiya D (2003) Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 9:219–225. doi:10.1093/molehr/gag031
McLaren A (1991) Sex determination in mammals. Oxf Rev Reprod Biol 13:1–33
Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351. doi:10.1006/dbio.2001.0269
Pepling ME, Spradling AC (1998) Female mouse germ cells form synchronously dividing cysts. Development 125:3323–3328
Perez GI, Trbovich AM, Gosden RG, Tilly JL (2000) Mitochondria and the death of oocytes. Nature 403:500–501. doi:10.1038/35000651
Alton M, Taketo T (2007) Switch from BAX-dependent to BAX-independent germ cell loss during the development of fetal mouse ovaries. J Cell Sci 120:417–424. doi:10.1242/jcs.03332
Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17:121–155. doi:10.1210/er.17.2.121
Richardson SJ, Senikas V, Nelson JF (1987) Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 65:1231–1237
Matova N, Cooley L (2001) Comparative aspects of animal oogenesis. Dev Biol 231:291–320. doi:10.1006/dbio.2000.0120
McGee EA, Hsueh AJ (2000) Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214. doi:10.1210/er.21.2.200
Morita Y, Perez GI, Maravei DV, Tilly KI, Tilly JL (1999) Targeted expression of Bcl-2 in mouse oocytes inhibits ovarian follicle atresia and prevents spontaneous and chemotherapy-induced oocyte apoptosis in vitro. Mol Endocrinol 13:841–850. doi:10.1210/me.13.6.841
Tilly JL (2001) Emerging technologies to control oocyte apoptosis are finally treading on fertile ground. ScientificWorldJournal 1:181–183
Hsueh AJ, Billig H, Tsafriri A (1994) Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev 15:707–724. doi:10.1210/er.15.6.707
Markstrom E, Svensson E, Shao R, Svanberg B, Billig H (2002) Survival factors regulating ovarian apoptosis—dependence on follicle differentiation. Reproduction 123:23–30. doi:10.1530/rep.0.1230023
Depalo R, Nappi L, Loverro G, Bettocchi S, Caruso ML, Valentini AM et al (2003) Evidence of apoptosis in human primordial and primary follicles. Hum Reprod 18:2678–2682. doi:10.1093/humrep/deg507
Murdoch WJ (1995) Programmed cell death in preovulatory ovine follicles. Biol Reprod 53:8–12. doi:10.1095/biolreprod53.1.8
Dharmarajan AM, Goodman SB, Atiya N, Parkinson SP, Lareu RR, Tilly KI et al (2004) Role of apoptosis in functional luteolysis in the pregnant rabbit corpus luteum: evidence of a role for placental-derived factors in promoting luteal cell survival. Apoptosis 9:807–814. doi:10.1023/B:APPT.0000045783.71178.c4
Tilly JL, Kowalski KI, Johnson AL, Hsueh AJ (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 129:2799–2801
D’Herde K, De Prest B, Roels F (1996) Subtypes of active cell death in the granulosa of ovarian atretic follicles in the quail (Coturnix coturnix japonica). Reprod Nutr Dev 36:175–189. doi:10.1051/rnd:19960203
Quatacker JR (1971) Formation of autophagic vacuoles during human corpus luteum involution. Z Zellforsch Mikrosk Anat 122:479–487. doi:10.1007/BF00936082
Tilly JL, Billig H, Kowalski KI, Hsueh AJ (1992) Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol Endocrinol 6:1942–1950. doi:10.1210/me.6.11.1942
Tilly JL (1996) Apoptosis and ovarian function. Rev Reprod 1:162–172. doi:10.1530/ror.0.0010162
Nezis IP, Stravopodis DJ, Papassideri I, Robert-Nicoud M, Margaritis LH (2002) Dynamics of apoptosis in the ovarian follicle cells during the late stages of Drosophila oogenesis. Cell Tissue Res 307:401–409. doi:10.1007/s00441-001-0498-3
Bellairs R (1964) Biological aspects of the yolk of the hen’s egg. Adv Morphog 4:217–272
Press N (1964) An unusual organelle in avian ovaries. J Ultrastruct Res 10:528–546. doi:10.1016/S0022-5320(64)80027-7
Schjeide OA, Galey F, Grellert EA, I-San Lin R, De Vellis J, Mead JF (1970) Macromolecules in oocyte maturation. Biol Reprod 2(Suppl 2):14–43. doi:10.1095/biolreprod2.Supplement_2.14
Schjeide OA, Hanzely L, Holshouser SJ, Briles WE (1974) Production and fates of unique organelles (transosomes) in ovarian follicles of Gallus domesticus under various conditions. Cell Tissue Res 156:47–59. doi:10.1007/BF00220101
Schjeide OA, Kancheva L, Hanzely L, Briles WE (1975) Production and fates of unique organelles (transosomes) in ovarian follicles of Gallus domesticus under various conditions. II. Cell Tissue Res 163:63–79. doi:10.1007/BF00218591
Paulson J, Rosenberg MD (1972) The function and transposition of lining bodies in developing avian oocytes. J Ultrastruct Res 40:25–43. doi:10.1016/S0022-5320(72)80020-0
Buccione R, Schroeder AC, Eppig JJ (1990) Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod 43:543–547. doi:10.1095/biolreprod43.4.543
Spradling AC (2004) Stem cells: more like a man. Nature 428:133–134. doi:10.1038/428133b
Kirilly D, Xie T (2007) The Drosophila ovary: an active stem cell community. Cell Res 17:15–25. doi:10.1038/sj.cr.7310123
Butler H, Juma MB (1970) Oogenesis in an adult prosimian. Nature 226:552–553. doi:10.1038/226552a0
Johnson J, Skaznik-Wikiel M, Lee HJ, Niikura Y, Tilly JC, Tilly JL (2005) Setting the record straight on data supporting postnatal oogenesis in female mammals. Cell Cycle 4:1471–1477
Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK (2006) Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction 132:95–109. doi:10.1530/rep.1.01128
Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R et al (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300:1251–1256. doi:10.1126/science.1083452
Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ (2004) Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427:148–154. doi:10.1038/nature02247
Toyooka Y, Tsunekawa N, Akasu R, Noce T (2003) Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci USA 100:11457–11462. doi:10.1073/pnas.1932826100
Daley GQ (2007) Gametes from embryonic stem cells: a cup half empty or half full? Science 316:409–410. doi:10.1126/science.1138772
Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Andersen CY et al (2005) On regenerating the ovary and generating controversy. Cell 122:821–822. doi:10.1016/j.cell.2005.09.004
Byskov AG, Faddy MJ, Lemmen JG, Andersen CY (2005) Eggs forever? Differentiation 73:438–446. doi:10.1111/j.1432-0436.2005.00045.x
Gougeon A (2005) Neo-oogenesis in the postnatal ovary: fantasy or reality? Gynecol Obstet Fertil 33:819–823. doi:10.1016/j.gyobfe.2005.07.029
Bukovsky A, Svetlikova M, Caudle MR (2005) Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol 3:17. doi:10.1186/1477-7827-3-17
Mandl AM, Zuckerman S (1951) The effect of destruction of the germinal epithelium on the numbers of oocytes. J Endocrinol 7:103–111
Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD et al (2006) Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol 298:149–154. doi:10.1016/j.ydbio.2006.06.023
Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ (2006) Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature 441:1109–1114. doi:10.1038/nature04929
Lee HJ, Selesniemi K, Niikura Y, Niikura T, Klein R, Dombkowski DM et al (2007) Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol 25:3198–3204. doi:10.1200/JCO.2006.10.3028
Veitia RA, Gluckman E, Fellous M, Soulier J (2007) Recovery of female fertility after chemotherapy, irradiation, and bone marrow allograft: further evidence against massive oocyte regeneration by bone marrow-derived germline stem cells. Stem Cells 25:1334–1335. doi:10.1634/stemcells.2006-0770
Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL et al (2007) Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol 306:112–120. doi:10.1016/j.ydbio.2007.03.006
de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW et al (2004) Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod 70:419–424. doi:10.1095/biolreprod.103.015784
Ioannou JM (1967) Oogenesis in adult prosimians. J Embryol Exp Morphol 17:139–145
David GF, Anand Kumar TC, Baker TG (1974) Uptake of tritiated thymidine by primordial germinal cells in the ovaries of the adult slender loris. J Reprod Fertil 41:447–451. doi:10.1530/jrf.0.0410447
Zhang D, Fouad H, Zoma WD, Salama SA, Wentz MJ, Al-Hendy A (2008) Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Reprod Sci 15:139–146. doi:10.1177/1933719107310708
Vermande-Van Eck GJ (1956) Neo-ovogenesis in the adult monkey; consequences of atresia of ovocytes. Anat Rec 125:207–224. doi:10.1002/ar.1091250205
Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ et al (1996) Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 87:3045–3052
Gosden RG, Laing SC, Felicio LS, Nelson JF, Finch CE (1983) Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol Reprod 28:255–260. doi:10.1095/biolreprod28.2.255
Park SM, Schickel R, Peter ME (2005) Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol 17:610–616. doi:10.1016/j.ceb.2005.09.010
Quirk SM, Cowan RG, Joshi SG, Henrikson KP (1995) Fas antigen-mediated apoptosis in human granulosa/luteal cells. Biol Reprod 52:279–287. doi:10.1095/biolreprod52.2.279
Kondo H, Maruo T, Peng X, Mochizuki M (1996) Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicular regression and atresia. J Clin Endocrinol Metab 81:2702–2710. doi:10.1210/jc.81.7.2702
Guo MW, Xu JP, Mori E, Sato E, Saito S, Mori T (1997) Expression of Fas ligand in murine ovary. Am J Reprod Immunol 37:391–398
Kim JM, Boone DL, Auyeung A, Tsang BK (1998) Granulosa cell apoptosis induced at the penultimate stage of follicular development is associated with increased levels of Fas and Fas ligand in the rat ovary. Biol Reprod 58:1170–1176. doi:10.1095/biolreprod58.5.1170
Peng X, Maruo T, Matsuo H, Takekida S, Deguchi J (1998) Serum deprivation-induced apoptosis in cultured porcine granulosa cells is characterized by increased expression of p53 protein, Fas antigen and Fas ligand and by decreased expression of PCNA. Endocr J 45:247–253. doi:10.1507/endocrj.45.247
Porter DA, Harman RM, Cowan RG, Quirk SM (2001) Relationship of Fas ligand expression and atresia during bovine follicle development. Reproduction 121:561–566. doi:10.1530/rep.0.1210561
Sakamaki K, Yoshida H, Nishimura Y, Nishikawa S, Manabe N, Yonehara S (1997) Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol Reprod Dev 47:11–18. doi:10.1002/(SICI)1098-2795(199705)47:1<11::AID-MRD2>3.0.CO;2-T
Hakuno N, Koji T, Yano T, Kobayashi N, Tsutsumi O, Taketani Y et al (1996) Fas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 137:1938–1948. doi:10.1210/en.137.5.1938
Benifla JL, Sifer C, Bringuier AF, Blanc-Layrac G, Camus E, Madelenat P et al (2002) Induced apoptosis and expression of related proteins in granulosa cells from women undergoing IVF: a preliminary study. Hum Reprod 17:916–920. doi:10.1093/humrep/17.4.916
Quirk SM, Cowan RG, Harman RM (2006) The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle. J Endocrinol 189:441–453. doi:10.1677/joe.1.06549
Dharma SJ, Kelkar RL, Nandedkar TD (2003) Fas and Fas ligand protein and mRNA in normal and atretic mouse ovarian follicles. Reproduction 126:783–789. doi:10.1530/rep.0.1260783
Chen Q, Yano T, Matsumi H, Osuga Y, Yano N, Xu J et al (2005) Cross-talk between Fas/Fas ligand system and nitric oxide in the pathway subserving granulosa cell apoptosis: a possible regulatory mechanism for ovarian follicle atresia. Endocrinology 146:808–815. doi:10.1210/en.2004-0579
Jiang JY, Cheung CK, Wang Y, Tsang BK (2003) Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front Biosci 8:d222–d237. doi:10.2741/949
Matsuda-Minehata F, Inoue N, Goto Y, Manabe N (2006) The regulation of ovarian granulosa cell death by pro- and anti-apoptotic molecules. J Reprod Dev 52:695–705. doi:10.1262/jrd.18069
Witty JP, Bridgham JT, Johnson AL (1996) Induction of apoptotic cell death in hen granulosa cells by ceramide. Endocrinology 137:5269–5277. doi:10.1210/en.137.12.5269
Kaipia A, Chun SY, Eisenhauer K, Hsueh AJ (1996) Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology 137:4864–4870. doi:10.1210/en.137.11.4864
Xiao CW, Asselin E, Tsang BK (2002) Nuclear factor kappaB-mediated induction of Flice-like inhibitory protein prevents tumor necrosis factor alpha-induced apoptosis in rat granulosa cells. Biol Reprod 67:436–441. doi:10.1095/biolreprod67.2.436
Xiao CW, Ash K, Tsang BK (2001) Nuclear factor-kappaB-mediated X-linked inhibitor of apoptosis protein expression prevents rat granulosa cells from tumor necrosis factor alpha-induced apoptosis. Endocrinology 142:557–563. doi:10.1210/en.142.2.557
Bridgham JT, Johnson AL (2002) Avian TVB (DR5-like) death receptor expression in hen ovarian follicles. Biochem Biophys Res Commun 291:226–232. doi:10.1006/bbrc.2002.6429
Wada S, Manabe N, Nakayama M, Inou N, Matsui T, Miyamoto H (2002) TRAIL-decoy receptor 1 plays inhibitory role in apoptosis of granulosa cells from pig ovarian follicles. J Vet Med Sci 64:435–439. doi:10.1292/jvms.64.435
Johnson AL, Ratajczak C, Haugen MJ, Liu HK, Woods DC (2007) Tumor necrosis factor-related apoptosis inducing ligand expression and activity in hen granulosa cells. Reproduction 133:609–616. doi:10.1530/REP-06-0287
Johnson AL, Bridgham JT (2002) Caspase-mediated apoptosis in the vertebrate ovary. Reproduction 124:19–27. doi:10.1530/rep.0.1240019
Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA et al (2001) Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology 142:2468–2480. doi:10.1210/en.142.6.2468
Boone DL, Tsang BK (1998) Caspase-3 in the rat ovary: localization and possible role in follicular atresia and luteal regression. Biol Reprod 58:1533–1539. doi:10.1095/biolreprod58.6.1533
Matsui T, Manabe N, Goto Y, Inoue N, Nishihara S, Miyamoto H (2003) Expression and activity of Apaf1 and caspase-9 in granulosa cells during follicular atresia in pig ovaries. Reproduction 126:113–120. doi:10.1530/rep.0.1260113
Robles R, Tao XJ, Trbovich AM, Maravel DV, Nahum R, Perez GI et al (1999) Localization, regulation and possible consequences of apoptotic protease-activating factor-1 (Apaf-1) expression in granulosa cells of the mouse ovary. Endocrinology 140:2641–2644. doi:10.1210/en.140.6.2641
D’Herde K, De Prest B, Mussche S, Schotte P, Beyaert R, Coster RV et al (2000) Ultrastructural localization of cytochrome c in apoptosis demonstrates mitochondrial heterogeneity. Cell Death Differ 7:331–337. doi:10.1038/sj.cdd.4400655
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A et al (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12:1304–1314. doi:10.1101/gad.12.9.1304
Morita Y, Maravei DV, Bergeron L, Wang S, Perez GI, Tsutsumi O et al (2001) Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia-mutated (Atm) gene function. Cell Death Differ 8:614–620. doi:10.1038/sj.cdd.4400845
Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A et al (2000) Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med 6:1109–1114. doi:10.1038/80442
Kim MR, Tilly JL (2004) Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochim Biophys Acta 1644:205–210. doi:10.1016/j.bbamcr.2003.10.012
Kugu K, Ratts VS, Piquette GN, Tilly KI, Tao XJ, Martimbeau S et al (1998) Analysis of apoptosis and expression of bcl-2 gene family members in the human and baboon ovary. Cell Death Differ 5:67–76. doi:10.1038/sj.cdd.4400316
Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL (1999) Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21:200–203. doi:10.1038/5985
Greenfeld CR, Babus JK, Furth PA, Marion S, Hoyer PB, Flaws JA (2007) BAX is involved in regulating follicular growth, but is dispensable for follicle atresia in adult mouse ovaries. Reproduction 133:107–116. doi:10.1530/REP-06-0144
Flaws JA, Hirshfield AN, Hewitt JA, Babus JK, Furth PA (2001) Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol Reprod 64:1153–1159. doi:10.1095/biolreprod64.4.1153
Hsu SY, Lai RJ, Finegold M, Hsueh AJ (1996) Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology 137:4837–4843. doi:10.1210/en.137.11.4837
Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL (1995) Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 136:3665–3668. doi:10.1210/en.136.8.3665
Hsu SY, Hsueh AJ (2000) Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev 80:593–614
Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ (1997) Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA 94:12401–12406. doi:10.1073/pnas.94.23.12401
Kaipia A, Hsu SY, Hsueh AJ (1997) Expression and function of a proapoptotic Bcl-2 family member Bcl-XL/Bcl-2-associated death promoter (BAD) in rat ovary. Endocrinology 138:5497–5504. doi:10.1210/en.138.12.5497
Craig J, Orisaka M, Wang H, Orisaka S, Thompson W, Zhu C et al (2007) Gonadotropin and intra-ovarian signals regulating follicle development and atresia: the delicate balance between life and death. Front Biosci 12:3628–3639. doi:10.2741/2339
Wang H, Tsang BK (2007) Nodal signalling and apoptosis. Reproduction 133:847–853. doi:10.1530/REP-07-0053
Boone DL, Carnegie JA, Rippstein PU, Tsang BK (1997) Induction of apoptosis in equine chorionic gonadotropin (eCG)-primed rat ovaries by anti-eCG antibody. Biol Reprod 57:420–427. doi:10.1095/biolreprod57.2.420
Bill CH 2nd, Greenwald GS (1981) Acute gonadotropin deprivation. I. A model for the study of follicular atresia. Biol Reprod 24:913–921. doi:10.1095/biolreprod24.4.913
Nahum R, Beyth Y, Chun SY, Hsueh AJ, Tsafriri A (1996) Early onset of deoxyribonucleic acid fragmentation during atresia of preovulatory ovarian follicles in rats. Biol Reprod 55:1075–1080. doi:10.1095/biolreprod55.5.1075
Kim JM, Yoon YD, Tsang BK (1999) Involvement of the Fas/Fas ligand system in p53-mediated granulosa cell apoptosis during follicular development and atresia. Endocrinology 140:2307–2317. doi:10.1210/en.140.5.2307
Hughes FM Jr, Gorospe WC (1991) Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology 129:2415–2422
Wang Y, Asselin E, Tsang BK (2002) Involvement of transforming growth factor alpha in the regulation of rat ovarian X-linked inhibitor of apoptosis protein expression and follicular growth by follicle-stimulating hormone. Biol Reprod 66:1672–1680. doi:10.1095/biolreprod66.6.1672
Mussche S, D’Herde K (2001) Contribution of progesterone, follicle stimulating hormone and glucocorticoids in survival of serum-free cultured granulosa cell explants. J Endocrinol 169:321–331. doi:10.1677/joe.0.1690321
Orisaka M, Orisaka S, Jiang JY, Craig J, Wang Y, Kotsuji F et al (2006) Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol 20:2456–2468. doi:10.1210/me.2005-0357
Wang H, Jiang JY, Zhu C, Peng C, Tsang BK (2006) Role and regulation of nodal/activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol Endocrinol 20:2469–2482. doi:10.1210/me.2005-0446
Zwain IH, Amato P (2001) cAMP-induced apoptosis in granulosa cells is associated with up-regulation of P53 and bax and down-regulation of clusterin. Endocr Res 27:233–249. doi:10.1081/ERC-100107184
Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR et al (1996) Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 10:903–918. doi:10.1210/me.10.7.903
Zhou J, Kumar TR, Matzuk MM, Bondy C (1997) Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol 11:1924–1933. doi:10.1210/me.11.13.1924
Chun SY, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsueh AJ (1994) Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology 135:1845–1853. doi:10.1210/en.135.5.1845
Guthrie HD, Garrett WM, Cooper BS (1998) Follicle-stimulating hormone and insulin-like growth factor-I attenuate apoptosis in cultured porcine granulosa cells. Biol Reprod 58:390–396. doi:10.1095/biolreprod58.2.390
Mao J, Smith MF, Rucker EB, Wu GM, McCauley TC, Cantley TC et al (2004) Effect of epidermal growth factor and insulin-like growth factor I on porcine preantral follicular growth, antrum formation, and stimulation of granulosal cell proliferation and suppression of apoptosis in vitro. J Anim Sci 82:1967–1975
Geisthovel F, Moretti-Rojas I, Asch RH, Rojas FJ (1989) Expression of insulin-like growth factor-II (IGF-II) messenger ribonucleic acid (mRNA), but not IGF-I mRNA, in human preovulatory granulosa cells. Hum Reprod 4:899–902
Zhou J, Bondy C (1993) Anatomy of the human ovarian insulin-like growth factor system. Biol Reprod 48:467–482. doi:10.1095/biolreprod48.3.467
Thierry van Dessel HJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH et al (1996) Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab 81:1224–1231. doi:10.1210/jc.81.3.1224
Adashi EY (1994) Regulation of intrafollicular IGFBPs: possible relevance to ovarian follicular selection. In: Baxter RC, Gluckman PD, Rosenfeld RO (eds) The insulin-like growth factors and their regulatory proteins. Excerpta medica international congress series 1056. Elsevier Science BV, Amsterdam, pp 341–419
Froment P, Seurin D, Hembert S, Levine JE, Pisselet C, Monniaux D et al (2002) Reproductive abnormalities in human IGF binding protein-1 transgenic female mice. Endocrinology 143:1801–1808. doi:10.1210/en.143.5.1801
Huang H, Rajkumar K, Murphy LJ (1997) Reduced fecundity in insulin-like growth factor-binding protein-1 transgenic mice. Biol Reprod 56:284–289. doi:10.1095/biolreprod56.1.284
Mazerbourg S, Bondy CA, Zhou J, Monget P (2003) The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles? A comparative species study. Reprod Domest Anim 38:247–258. doi:10.1046/j.1439-0531.2003.00440.x
Saito H, Kaneko T, Takahashi T, Kawachiya S, Saito T, Hiroi M (2000) Hyaluronan in follicular fluids and fertilization of oocytes. Fertil Steril 74:1148–1152. doi:10.1016/S0015-0282(00)01586-7
Turley EA (1992) Hyaluronan and cell locomotion. Cancer Metastasis Rev 11:21–30. doi:10.1007/BF00047600
Sato E, Ishibashi T, Koide SS (1987) Prevention of spontaneous degeneration of mouse oocytes in culture by ovarian glycosaminoglycans. Biol Reprod 37:371–376. doi:10.1095/biolreprod37.2.371
Miyano T, Hiro-Oka RE, Kano K, Miyake M, Kusunoki H, Kato S (1994) Effects of hyaluronic acid on the development of 1- and 2-cell porcine embryos to the blastocyst stage in vitro. Theriogenology 41:1299–1305. doi:10.1016/0093-691X(94)90488-5
Ohta N, Saito H, Kuzumaki T, Takahashi T, Ito MM, Saito T et al (1999) Expression of CD44 in human cumulus and mural granulosa cells of individual patients in in-vitro fertilization programmes. Mol Hum Reprod 5:22–28. doi:10.1093/molehr/5.1.22
Kaneko T, Saito H, Toya M, Satio T, Nakahara K, Hiroi M (2000) Hyaluronic acid inhibits apoptosis in granulosa cells via CD44. J Assist Reprod Genet 17:162–167. doi:10.1023/A:1009470206468
Adashi EY (1992) The potential relevance of cytokines to ovarian physiology. J Steroid Biochem Mol Biol 43:439–444. doi:10.1016/0960-0760(92)90082-T
Adashi EY, Resnick CE, Hurwitz A, Ricciarelli E, Hernandez ER, Roberts CT et al (1991) Insulin-like growth factors: the ovarian connection. Hum Reprod 6:1213–1219
Krysko DV, Leybaert L, Vandenabeele P, D’Herde K (2005) Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10:459–469. doi:10.1007/s10495-005-1875-2
Veitch GI, Gittens JE, Shao Q, Laird DW, Kidder GM (2004) Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J Cell Sci 117:2699–2707. doi:10.1242/jcs.01124
Gittens JE, Barr KJ, Vanderhyden BC, Kidder GM (2005) Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J Cell Sci 118:113–122. doi:10.1242/jcs.01587
Tong D, Li TY, Naus KE, Bai D, Kidder GM (2007) In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci 120:4016–4024. doi:10.1242/jcs.011775
Li TY, Colley D, Barr KJ, Yee SP, Kidder GM (2007) Rescue of oogenesis in Cx37-null mutant mice by oocyte-specific replacement with Cx43. J Cell Sci 120:4117–4125. doi:10.1242/jcs.03488
Kidder GM, Mhawi AA (2002) Gap junctions and ovarian folliculogenesis. Reproduction 123:613–620. doi:10.1530/rep.0.1230613
Simon AM, Goodenough DA, Li E, Paul DL (1997) Female infertility in mice lacking connexin 37. Nature 385:525–529. doi:10.1038/385525a0
Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF (2000) Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 226:167–179. doi:10.1006/dbio.2000.9863
Sugiura K, Pendola FL, Eppig JJ (2005) Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 279:20–30. doi:10.1016/j.ydbio.2004.11.027
Sasson R, Shinder V, Dantes A, Land A, Amsterdam A (2003) Activation of multiple signal transduction pathways by glucocorticoids: protection of ovarian follicular cells against apoptosis. Biochem Biophys Res Commun 311:1047–1056. doi:10.1016/j.bbrc.2003.10.097
Sasson R, Amsterdam A (2002) Stimulation of apoptosis in human granulosa cells from in vitro fertilization patients and its prevention by dexamethasone: involvement of cell contact and bcl-2 expression. J Clin Endocrinol Metab 87:3441–3451. doi:10.1210/jc.87.7.3441
D’Herde K, Leybaert L (1997) Intracellular free calcium related to apoptotic cell death in quail granulosa cell sheets kept in serum-free culture. Cell Death Differ 4:59–65. doi:10.1038/sj.cdd.4400201
Krysko DV, Mussche S, Leybaert L, D’Herde K (2004) Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells. J Histochem Cytochem 52:1199–1207. doi:10.1369/jhc.3A6227.2004
Cheng Y, Inoue N, Matsuda-Minehata F, Goto Y, Maeda A, Manabe N (2005) Changes in expression and localization of connexin 43 mRNA and protein in porcine ovary granulosa cells during follicular atresia. J Reprod Dev 51:627–637. doi:10.1262/jrd.17035
Kaiser N, Edelman IS (1977) Calcium dependence of glucocorticoid-induced lymphocytolysis. Proc Natl Acad Sci USA 74:638–642. doi:10.1073/pnas.74.2.638
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4:552–565. doi:10.1038/nrm1150
McConkey DJ (1996) The role of calcium in the regulation of apoptosis. Scanning Microsc 10:777–793; discussion 793–774
D’Herde K, Leybaert L (1998) Apoptotic granulosa cells have moderately increased intracellular free calcium during phosphatidylserine exposure and a normal resting calcium level during DNA fragmentation. Apoptosis 3:337–343
Mussche S, Leybaert L, D’Herde K (2000) First and second messenger role of calcium. Survival versus apoptosis in serum-free cultured granulosa explants. Ann NY Acad Sci 926:101–115
Velentzas AD, Nezis IP, Stravopodis DJ, Papassideri IS, Margaritis LH (2007) Apoptosis and autophagy function cooperatively for the efficacious execution of programmed nurse cell death during Drosophila virilis oogenesis. Autophagy 3:130–132
Duerrschmidt N, Zabirnyk O, Nowicki M, Ricken A, Hmeidan FA, Blumenauer V et al (2006) Lectin-like oxidized low-density lipoprotein receptor-1-mediated autophagy in human granulosa cells as an alternative of programmed cell death. Endocrinology 147:3851–3860. doi:10.1210/en.2006-0088
Elder KCJ (2007) Human preimplantation embryo selection, 1st edn. Informa Healthcare
Ebner T, Moser M, Sommergruber M, Tews G (2003) Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update 9:251–262. doi:10.1093/humupd/dmg021
Kimura N, Hoshino Y, Totsukawa K, Sato E (2007) Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl 63:327–342
Attaran M, Frasor J, Mascha E, Radwanska E, Rawlins RG (1998) The relationship of human granulosa-lutein cell proliferative index to follicular diameter and serum estradiol. Obstet Gynecol 91:449–453. doi:10.1016/S0029-7844(97)00707-2
Nakahara K, Saito H, Saito T, Ito M, Ohta N, Sakai N et al (1997) Incidence of apoptotic bodies in membrana granulosa of the patients participating in an in vitro fertilization program. Fertil Steril 67:302–308. doi:10.1016/S0015-0282(97)81915-2
Nakahara K, Saito H, Saito T, Ito M, Ohta N, Takahashi T et al (1997) The incidence of apoptotic bodies in membrana granulosa can predict prognosis of ova from patients participating in in vitro fertilization programs. Fertil Steril 68:312–317. doi:10.1016/S0015-0282(97)81521-X
Suh CS, Jee BC, Choi YM, Kim JG, Lee JY, Moon SY et al (2002) Prognostic implication of apoptosis in human luteinized granulosa cells during IVF-ET. J Assist Reprod Genet 19:209–214. doi:10.1023/A:1015319617598
Idil M, Cepni I, Demirsoy G, Ocal P, Salihoglu F, Senol H et al (2004) Does granulosa cell apoptosis have a role in the etiology of unexplained infertility? Eur J Obstet Gynecol Reprod Biol 112:182–184. doi:10.1016/S0301-2115(03)00365-8
Piquette GN, Tilly JL, Prichard LE, Simon C, Polan ML (1994) Detection of apoptosis in human and rat ovarian follicles. J Soc Gynecol Investig 1:297–301
Clavero A, Castilla JA, Nunez AI, Garcia-Pena ML, Maldonado V, Fontes J et al (2003) Apoptosis in human granulosa cells after induction of ovulation in women participating in an intracytoplasmic sperm injection program. Eur J Obstet Gynecol Reprod Biol 110:181–185. doi:10.1016/S0301-2115(03)00243-4
Oosterhuis GJ, Michgelsen HW, Lambalk CB, Schoemaker J, Vermes I (1998) Apoptotic cell death in human granulosa-lutein cells: a possible indicator of in vitro fertilization outcome. Fertil Steril 70:747–749. doi:10.1016/S0015-0282(98)00266-0
Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW (2001) Cumulus cells apoptosis as an indicator to predict the quality of oocytes and the outcome of IVF-ET. J Assist Reprod Genet 18:490–498. doi:10.1023/A:1016649026353
Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P et al (2005) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 12(Suppl 2):1463–1467. doi:10.1038/sj.cdd.4401724
Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P (2008) Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44:205–221. doi:10.1016/j.ymeth.2007.12.001
Amsterdam A, Koch Y, Lieberman ME, Lindner HR (1975) Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol 67:894–900. doi:10.1083/jcb.67.3.894
Lawrence TS, Dekel N, Beers WH (1980) Binding of human chorionic gonadotropin by rat cumuli oophori and granulosa cells: a comparative study. Endocrinology 106:1114–1118
Dirnfeld M, Goldman S, Gonen Y, Koifman M, Lissak A, Kraiem Z et al (1993) Functional differentiation in progesterone secretion by granulosa versus cumulus cells in the human preovulatory follicle and the effect of different induction of ovulation protocols. Fertil Steril 60:1025–1030
Billig H, Furuta I, Hsueh AJ (1994) Gonadotropin-releasing hormone directly induces apoptotic cell death in the rat ovary: biochemical and in situ detection of deoxyribonucleic acid fragmentation in granulosa cells. Endocrinology 134:245–252. doi:10.1210/en.134.1.245
Nakahara K, Saito H, Saito T, Ito M, Ohta N, Takahashi T et al (1998) Ovarian fecundity in patients with endometriosis can be estimated by the incidence of apoptotic bodies. Fertil Steril 69:931–935. doi:10.1016/S0015-0282(98)00038-7
Tilly JL (1997) Apoptosis and the ovary: a fashionable trend or food for thought? Fertil Steril 67:226–228. doi:10.1016/S0015-0282(97)81901-2
Saito H, Saito T, Kaneko T, Sasagawa I, Kuramoto T, Hiroi M (2000) Relatively poor oocyte quality is an indication for intracytoplasmic sperm injection. Fertil Steril 73:465–469. doi:10.1016/S0015-0282(99)00547-6
Host E, Gabrielsen A, Lindenberg S, Smidt-Jensen S (2002) Apoptosis in human cumulus cells in relation to zona pellucida thickness variation, maturation stage, and cleavage of the corresponding oocyte after intracytoplasmic sperm injection. Fertil Steril 77:511–515. doi:10.1016/S0015-0282(01)03006-0
Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H (2002) Eight-hydroxy-2’-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril 77:1184–1190. doi:10.1016/S0015-0282(02)03103-5
Al-Inany HG, Abou-Setta AM, Aboulghar M (2007) Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reprod Biomed Online 14:640–649
Breckwoldt M, Selvaraj N, Aharoni D, Barash A, Segal I, Insler V et al (1996) Expression of Ad4-BP/cytochrome P450 side chain cleavage enzyme and induction of cell death in long-term cultures of human granulosa cells. Mol Hum Reprod 2:391–400. doi:10.1093/molehr/2.6.391
Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC (2007) Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril 87:542–546. doi:10.1016/j.fertnstert.2006.06.059
Wynn P, Picton HM, Krapez JA, Rutherford AJ, Balen AH, Gosden RG (1998) Pretreatment with follicle stimulating hormone promotes the numbers of human oocytes reaching metaphase II by in-vitro maturation. Hum Reprod 13:3132–3138. doi:10.1093/humrep/13.11.3132
Mikkelsen AL, Smith SD, Lindenberg S (1999) In-vitro maturation of human oocytes from regularly menstruating women may be successful without follicle stimulating hormone priming. Hum Reprod 14:1847–1851. doi:10.1093/humrep/14.7.1847
Neveu S, Hedon B, Bringer J, Chinchole JM, Arnal F, Humeau C et al (1987) Ovarian stimulation by a combination of a gonadotropin-releasing hormone agonist and gonadotropins for in vitro fertilization. Fertil Steril 47:639–643
Ashkenazi J, Dicker D, Feldberg D, Goldman GA, Yeshaya A, Goldman JA (1989) The value of GnRH analogue therapy in IVF in women with unexplained infertility. Hum Reprod 4:667–669
Zhao S, Saito H, Wang X, Saito T, Kaneko T, Hiroi M (2000) Effects of gonadotropin-releasing hormone agonist on the incidence of apoptosis in porcine and human granulosa cells. Gynecol Obstet Invest 49:52–56. doi:10.1159/000010213
Kaneko T, Saito H, Takahashi T, Ohta N, Saito T, Hiroi M (2000) Effects of controlled ovarian hyperstimulation on oocyte quality in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet 17:580–585. doi:10.1023/A:1026439409584
Toya M, Saito H, Ohta N, Saito T, Kaneko T, Hiroi M (2000) Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril 73:344–350. doi:10.1016/S0015-0282(99)00507-5
Seifer DB, Gardiner AC, Ferreira KA, Peluso JJ (1996) Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril 66:593–598
Sadraie SH, Saito H, Kaneko T, Saito T, Hiroi M (2000) Effects of aging on ovarian fecundity in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet 17:168–173. doi:10.1023/A:1009422323306
Baka S, Malamitsi-Puchner A (2006) Novel follicular fluid factors influencing oocyte developmental potential in IVF: a review. Reprod Biomed Online 12:500–506
Wang Q, Sun QY (2007) Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev 19:1–12. doi:10.1071/RD06103
Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, Frydman R et al (2007) Anti-Mullerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab 92:1796–1802. doi:10.1210/jc.2006-1053
Tavmergen E, Tavmergen EN, Capanoglu R (1992) Do analogues of gonadotrophin releasing hormone influence follicular fluid steroid levels, oocyte maturity and fertilization rates? Hum Reprod 7:479–482
Kamel MA, Zabel G, Bernart W, Neulen J, Breckwoldt M (1994) Comparison between prolactin, gonadotrophins and steroid hormones in serum and follicular fluid after stimulation with gonadotrophin-releasing hormone agonists and human menopausal gonadotrophin for an in-vitro fertilization programme. Hum Reprod 9:1803–1806
Sugino N, Takiguchi S, Ono M, Tamura H, Shimamura K, Nakamura Y et al (1996) Nitric oxide concentrations in the follicular fluid and apoptosis of granulosa cells in human follicles. Hum Reprod 11:2484–2487
Amsterdam A, Dantes A, Selvaraj N, Aharoni D (1997) Apoptosis in steroidogenic cells: structure-function analysis. Steroids 62:207–211. doi:10.1016/S0039-128X(96)00182-1
Spicer LJ (2004) Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles: a control mechanism for selection of dominant follicles. Biol Reprod 70:1223–1230. doi:10.1095/biolreprod.103.021006
Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC (1999) The insulin-related ovarian regulatory system in health and disease. Endocr Rev 20:535–582. doi:10.1210/er.20.4.535
Bencomo E, Perez R, Arteaga MF, Acosta E, Pena O, Lopez L et al (2006) Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril 85:474–480. doi:10.1016/j.fertnstert.2005.08.014
Duleba AJ, Spaczynski RZ, Olive DL (1998) Insulin and insulin-like growth factor I stimulate the proliferation of human ovarian theca-interstitial cells. Fertil Steril 69:335–340. doi:10.1016/S0015-0282(97)00473-1
Nicholas B, Alberio R, Fouladi-Nashta AA, Webb R (2005) Relationship between low-molecular-weight insulin-like growth factor-binding proteins, caspase-3 activity, and oocyte quality. Biol Reprod 72:796–804. doi:10.1095/biolreprod.104.036087
Salustri A, Yanagishita M, Underhill CB, Laurent TC, Hascall VC (1992) Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol 151:541–551. doi:10.1016/0012-1606(92)90192-J
Suchanek E, Simunic V, Juretic D, Grizelj V (1994) Follicular fluid contents of hyaluronic acid, follicle-stimulating hormone and steroids relative to the success of in vitro fertilization of human oocytes. Fertil Steril 62:347–352
Ohta N, Saito H, Kaneko T, Yoshida M, Takahashi T, Saito T et al (2001) Soluble CD44 in human ovarian follicular fluid. J Assist Reprod Genet 18:21–25. doi:10.1023/A:1026494528415
Onalan G, Selam B, Onalan R, Ceyhan T, Cincik M, Pabuccu R (2006) Serum and follicular fluid levels of soluble Fas and soluble Fas ligand in IVF cycles. Eur J Obstet Gynecol Reprod Biol 125:85–91. doi:10.1016/j.ejogrb.2005.08.002
Knight PG, Glister C (2006) TGF-beta superfamily members and ovarian follicle development. Reproduction 132:191–206. doi:10.1530/rep.1.01074
Chang CL, Wang TH, Horng SG, Wu HM, Wang HS, Soong YK (2002) The concentration of inhibin B in follicular fluid: relation to oocyte maturation and embryo development. Hum Reprod 17:1724–1728. doi:10.1093/humrep/17.7.1724
Fried G, Remaeus K, Harlin J, Krog E, Csemiczky G, Aanesen A et al (2003) Inhibin B predicts oocyte number and the ratio IGF-I/IGFBP-1 may indicate oocyte quality during ovarian hyperstimulation for in vitro fertilization. J Assist Reprod Genet 20:167–176. doi:10.1023/A:1023656225053
Hall JE, Welt CK, Cramer DW (1999) Inhibin A and inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Hum Reprod 14:409–415. doi:10.1093/humrep/14.2.409
Molina JR, Barton DL, Loprinzi CL (2005) Chemotherapy-induced ovarian failure: manifestations and management. Drug Saf 28:401–416. doi:10.2165/00002018-200528050-00004
Nippita TA, Baber RJ (2007) Premature ovarian failure: a review. Climacteric 10:11–22. doi:10.1080/13697130601135672
Meirow D, Nugent D (2001) The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 7:535–543. doi:10.1093/humupd/7.6.535
Lo Presti A, Ruvolo G, Gancitano RA, Cittadini E (2004) Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol 113(Suppl 1):S33–S40. doi:10.1016/j.ejogrb.2003.11.008
Thibaud E, Rodriguez-Macias K, Trivin C, Esperou H, Michon J, Brauner R (1998) Ovarian function after bone marrow transplantation during childhood. Bone Marrow Transplant 21:287–290. doi:10.1038/sj.bmt.1701075
Swanton A, Child T (2005) Reproduction and ovarian ageing. J Br Menopause Soc 11:126–131. doi:10.1258/136218005775544200
Blatt J (1999) Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol 33:29–33. doi:10.1002/(SICI)1096-911X(199907)33:1<29::AID-MPO6>3.0.CO;2-2"
Sonmezer M, Oktay K (2004) Fertility preservation in female patients. Hum Reprod Update 10:251–266. doi:10.1093/humupd/dmh021
Oktem O, Oktay K (2007) Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110:2222–2229. doi:10.1002/cncr.23071
Chiarelli AM, Marrett LD, Darlington G (1999) Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964–1988 in Ontario, Canada. Am J Epidemiol 150:245–254
Wallace WH, Shalet SM, Hendry JH, Morris-Jones PH, Gattamaneni HR (1989) Ovarian failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol 62:995–998
Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM (2006) Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update 12:519–535. doi:10.1093/humupd/dml032
Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H et al (2007) The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol 130:148–155. doi:10.1016/j.ejogrb.2006.08.006
Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL (1997) Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 3:1228–1232. doi:10.1038/nm1197-1228
Jurisicova A, Lee HJ, D’Estaing SG, Tilly J, Perez GI (2006) Molecular requirements for doxorubicin-mediated death in murine oocytes. Cell Death Differ 13:1466–1474. doi:10.1038/sj.cdd.4401819
Reynolds T (1999) Cell death genes may hold clues to preserving fertility after chemotherapy. J Natl Cancer Inst 91:664–666. doi:10.1093/jnci/91.8.664
Ataya K, Ramahi-Ataya A (1993) Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol 7:229–235. doi:10.1016/0890-6238(93)90229-Z
Ataya K, Rao LV, Lawrence E, Kimmel R (1995) Luteinizing hormone-releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod 52:365–372. doi:10.1095/biolreprod52.2.365
Blumenfeld Z, Eckman A (2005) Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J Natl Cancer Inst Monogr 34:40–43. doi:10.1093/jncimonographs/lgi015
Blumenfeld Z (2007) How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 12:1044–1054. doi:10.1634/theoncologist.12-9-1044
Tilly JL, Kolesnick RN (2002) Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim Biophys Acta 1585:135–138
Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY et al (2001) Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet 28:355–360. doi:10.1038/ng575
Takai Y, Matikainen T, Jurisicova A, Kim MR, Trbovich AM, Fujita E et al (2007) Caspase-12 compensates for lack of caspase-2 and caspase-3 in female germ cells. Apoptosis 12:791–800. doi:10.1007/s10495-006-0022-z
Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A et al (2007) Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci USA 104:5229–5234. doi:10.1073/pnas.0608557104
Acknowledgements
Dmitri V. Krysko is supported by a postdoctoral fellowship from the BOF (Bijzonder Onderzoeksfonds 01P05807), Ghent University. We thank Dr. Amin Bredan for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krysko, D.V., Diez-Fraile, A., Criel, G. et al. Life and death of female gametes during oogenesis and folliculogenesis. Apoptosis 13, 1065–1087 (2008). https://doi.org/10.1007/s10495-008-0238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0238-1