Abstract
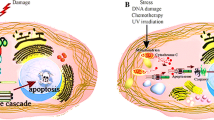
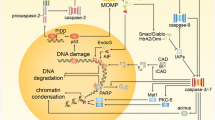
CHO cells were grown in the presence of 1 μ M CdCl2 and subjected to ATP-dependent replicative DNA synthesis after permeabilization. By decreasing the density of the cell culture replicative DNA synthesis was diminishing. At higher than 2 × 106 cell/ml concentration Cd had virtually no effect on the rate of DNA replication. Growth at higher cell concentrations could be supressed by increasing Cd concentration. After Cd treatment cells were synchronized by counterflow centrifugal elutriation. Cadmium toxicity on cell growth in early and mid S phase led to the accumulation of enlarged cells in late S phase. Flow cytometry showed increased cellular and nuclear sizes after Cd treatment. As the cells progressed through the S phase, 11 subpopulations of nuclear sizes were distinguished. Apoptotic chromatin changes were visualized by fluorescent microscopy in a cell cycle dependent manner. In the control untreated cells the main transitory forms of chromatin corresponded to those we have published earlier (veil-like, supercoiled chromatin, fibrous, ribboned structures, chromatin strings, elongated prechromosomes, precondensed chromosomes). Cadmium treatment caused: (a) the absence of decondensed veil-like structures and premature chromatin condensation in the form of apoptotic bodies in early S phase (2.2–2.4 average C-value), (b) the absence of fibrous structures, the lack of supercoiled chromatin, the appearance of uncoiled ribboned chromatin and perichromatin semicircles, in early mid S phase (2.5–2.9 C), (c) the presence of perichromatin fibrils and chromatin bodies in mid S phase (2.9–3.2 C), (d) early intra-nuclear inclusions, elongated forms of premature chromosomes, the extrusion and rupture of nuclear membrane later in mid S phase (3.3–3.4 C), (e) the exclusion of chromatin bodies and the formation of clusters of large-sized perichromatin granules in late S phase (3.5–3.8 C) and (f) large extensive disruptions and holes in the nuclear membrane and the clumping of incompletely folded chromosomes (3.8–4. C).
Similar content being viewed by others
References
Meplan C, Mann K, Hainaut P. Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J Biol Chem 1999; 274: 31663–31670.
el Azzouzi B, Tsangaris GT, Pellegrini O, Mauel Y, Benveniste J, Thomas Y. Cadmium induces apoptosis in a human T cell line. Toxicology 1994; 88: 127–139.
Cervera J, Alamar M, Matinez A, Renau-Piqueras J. Nuclear alterations induced by cadmium chloride and L-canavanine in HeLa S3 cells. Accumulation of perichromatin granules. J Ultrastruct Res 1983; 82: 241–263.
Ree K, Rugstad HE, Bakka A. Ultrastructural changes in the nucleus of a human epithelial cell line exposed to cytotoxic agents. The effect of PUVA and cadmium. Acta Pathol Microbiol Immunol Scand [A]. 1982; 90: 427–435.
Morselt AF, Peereboom-Stegeman JH, Jongstra-Spaapen EJ, James J. Investigation of the mechanism of cadmium toxicity at cellular level. I. A light microscopical study. Arch Toxicol 1983; 52: 91–97.
Aydin HH, Celik HA, Deveci R, et al. Characterization of the cellular response during apoptosis induction in cadmium-treated Hep G2 human hepatoma cells. Biol Trace Elem Res 2003; 95: 139–153.
Nagy G, Gacsi M, Rehak M, Basnakian AG, Klaisz M, Banfalvi G. Gamma irradiation-induced apoptosis in murine pre-B cells prevents the condensation of fibrillar chromatin in early S phase. Apoptosis 2004; 9: 765–776.
Banfalvi G, Poirier LA, Mikhailova M, Chou MW. Relationship of repair and replicative DNA synthesis to cell cycle in Chinese hamster ovary (CHO-K1). DNA Cell Biol 1997; 16: 1155–1160.
Offer H, Zurer I, Banfalvi G, et al. p53 modulates base excision activity in a cell cycle-specific manner after genotoxic stress. Cancer Res. 2001; 61: 88–96.
Basnakian AG, Banfalvi G, Sarkar N. Contribution of DNA polymerase δ to DNA replication in permeable CHO cells within nuclei of Chinese hamster ovary cells synchronized in S phase. Nucleic Acid Res. 1989; 17: 4757–4767.
Banfalvi G, Sooki-Toth A, Sarkar N, Csuzi S, Antoni F. Nascent DNA synthesized in reversibly permeable cells of mouse thymocytes. Eur J Biochem 1984; 139: 553–559.
Mascetti G, Carrara S, Vergani L. Relationship between chromatin compactness and dye uptake for in situ chromatin stained with DAPI. Cytometry 2001; 44: 113–119.
Gacsi M, Nagy G, Pinter G, Basnakian AG, Banfalvi G. Condensation of interphase chromatin in nuclei of Chinese hamster ovary (CHO-K1) cells. DNA Cell Biol 2005; 24: 43– 53.
Bell SW, Masters SK, Ingram P, Waters M, Shelburne JD. Ultrastructure and X-ray microanalysis of macrophages exposed to cadmium chloride. Scan Electron Microsc. 1979; 3: 111–121.
Hirano T, Ueda H, Kawahara A, Fujimoto S. Cadmium toxicity on cultured neonatal rat hepatocytes: Biochemical and ultrastructural analyses. Histol Histopathol 1991; 6: 127– 133.
Ord MJ, Bouffler SD, Chibber R. Cadmium induced changes in cell organelles: An ultrastructural study using cadmium sensitive and resistant muntjac fibroblast cell lines. Arch Toxicol 1988; 62: 133–145.
Gill TS, Pant JC. Erythrocytic and leukocytic responses to cadmium poisoning in a freshwater fish, Puntius conchonius Ham. Environ Res 1985; 36: 327–337.
Kimura Y, Sugimoto C, Matsukawa S, et al. Combined treatment of cisplatin and overexpression of caspase-activated deoxyribonuclease (CAD) promotes apoptosis in vitro and in vivo. Oral Oncol 2004; 40: 390–399.
Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ 2003; 10: 108–116.
Banfalvi G, Littlefield N, Hass B, et al. Effect of cadmium on the relationship between replicative and repair DNA synthesis in synchronized CHO cells. Eur J Biochem 2000; 267: 6580–6585.
Woo EJ, Kim YG, Kim MS, et al. Structural mechanism for inactivation and activation of CAD/DFF40 in the apoptotic pathway. Mol Cell 2004; 14: 531–539.
Banfalvi G, Mikhailova M, Poirier LA, Chou MW. Multiple Subphases of DNA Replication in Chinese Hamster Ovary (CHO-K1) Cells. DNA and Cell Biol 1997; 16: 1493–1498.
Rehak M, Csuka I, Szepessy E, Banfalvi G. Subphases of DNA Replication in Drosophila Cells. DNA and Cell Biol 2000; 19: 607–612.
Di Sant’Agnese PA, Jensen KD, Levin A, Miller RK. Placental toxicity of cadmium in the rat: An ultrastructural study. Placenta 1983; 4: 149–163.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banfalvi, G., Gacsi, M., Nagy, G. et al. Cadmium induced apoptotic changes in chromatin structure and subphases of nuclear growth during the cell cycle in CHO cells. Apoptosis 10, 631–642 (2005). https://doi.org/10.1007/s10495-005-1897-9
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-1897-9