Abstract
Mountain scree habitats are intermediate habitats between the base of the soil and the bedrock. They are composed of a network of small cracks and voids, and are commonly situated at the lower levels of scree slopes. Their environment is defined by empty spaces inside the scree, the absence of light and photoperiod, low temperature, and resource poor conditions. Soil arthropod communities, their trophic structure as well as their use of basal resources in mountain scree are little studied despite the fact that they are important components of these systems. Here, we investigate stable isotope ratios (15N/14N, 13C/12C) of oribatid mites (Oribatida, Acari) to understand their trophic niches and their variation with depth (50 and 75 cm) at two mountain scree sites (Cerdacul Stanciului, Marele Grohotis) in the Romanian Carpathians. Further, we used existing data to investigate the reproductive mode of the species in that habitat, as this may be related to resource availability. We hypothesized that trophic niches of oribatid mites will not differ between the two mountain scree regions but will be affected by depth. We furthermore hypothesized that due to the resource poor conditions oribatid mite species will span a narrow range of trophic levels, and that species are sexual rather than parthenogenetic. Our results showed that (1) oribatid mite trophic structure only slightly differed between the two sites indicating that the trophic ecology of oribatid mites in scree habitats is consistent and predictable, (2) oribatid mite trophic structure did not differ between the two studied soil depths indicating that the structure and availability of resources that were used by oribatid mites in deeper scree habitats varies little with depth, (3) oribatid mite species spanned only three trophic levels indicating that the habitat is rather resource poor, and (4) that all studied oribatid mite species were sexual supporting the view that resource poor conditions favour sexual reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mountain scree habitats are commonly found in scree slopes, where soil has not filled in the spaces. Scree represents an intermediate habitat between soil and bedrock. It is composed of a network of small cracks and voids, and usually is situated at the lower level of scree slopes (Juberthie et al. 1980; Juberthie 1983; Nitzu et al. 2014). Mountain scree habitats are characterised by little light and photoperiodicity, contrasting caves where at the entrance the presence of light allows vegetation development. Mountain scree habitats are characterized by very low availability of resources; however, the availability of resources may differ between mountain scree regions and it may also decline with depth (Culver and Pipan 2019). Resources enter this habitat by (1) water (hydrochoric transportation), (2) gravity (gravitational transportation), and (3) active animal migrations from the surface or from the deep hypogean domain (biochoric transportation) (Mammola et al. 2016). The limited availability of resources makes this habitat an ideal study site for understanding the trophic ecology and the use of basal resources of soil animals such as oribatid mites under resource poor conditions (Mumladze et al. 2015).
Oribatid mites (Oribatida, Acari) are an important component of the arthropod community in virtually all terrestrial ecosystems (Maraun and Scheu 2000). They feed on decomposing plant material and fungi, but also on lichens and living or dead animals (Walter and Proctor 1999; Schneider et al. 2004), representing the full decomposer community. Oribatid mites also are an ideal group to investigate the factors affecting soil animal community structure, including both density-independent (Lindberg and Bengtsson 2005; Badejo and Akinwole 2006; Bluhm et al. 2016) and density-dependent factors (Fischer et al. 2010; Caruso et al. 2013). Oribatid mite diversity varies among habitats (Schatz and Behan-Pelletier 2008), although the factors responsible for their variation in space are little understood (Maraun and Scheu 2000; Caruso et al. 2019). Oribatid mite communities of subterranean habitats such as mountain scree have been studied intensively (Skubała et al. 2013; Jiménez-Valverde et al. 2015; Nae and Băncilă 2017), but their trophic structure is little known.
Around 10% of oribatid mite species reproduce by parthenogenesis, i.e., thelytoky, which is much higher than in other invertebrate and vertebrate taxa except in bdelloid rotifers (Fischer et al. 2010). In some ecosystems, even more than 90% of the individuals of oribatid mites in soil reproduce via parthenogenesis (Norton and Palmer 1991; Maraun et al. 2003, 2012). By contrast, in caves and scree habitats both the number of sexual oribatid mite species and individuals are high, whereas their densities are low (Maraun et al. 2019). Presumably, resource limitation triggers the abundance and frequency of sexual taxa, especially in soil animals (Scheu and Drossel 2007).
Increasingly, trophic niches of animals are being analysed measuring natural variations in stable isotope ratios of nitrogen (15N/14N) and carbon (13C/12C). The method provides integrative insight into the trophic position of consumers and also into the use of basal resources in soil animal communities (Scheu and Falca 2000; Tiunov 2007; Maraun et al. 2011; Potapov et al. 2019). Consumers are enriched in 15N by an average of 3.4 δ units relative to their food source (DeNiro and Epstein 1981; Post 2002; Martinez del Rio et al. 2009). By contrast, fractionation of 13C is lower, averaging ~ 0.4 δ units per trophic level (Post 2002; Martinez del Rio et al. 2009), and therefore is of little use to determine the trophic structure of communities (Ponsard and Arditi 2000); however, in particular in soil it allows to trace the use of basal resources of consumers (Albers et al. 2006; Pollierer et al. 2009; Melguizo-Ruiz et al. 2017; Potapov et al. 2019).
The present study investigates for the first time the trophic structure of oribatid mite species in mountain scree using stable isotopes (13C, 15N). Additionally, we use existing data to investigate the reproductive mode in this particular subterranean habitat. We hypothesized that (1) the trophic niches of oribatid mites differ little in space, i.e., between scree habitats as environmental conditions in scree habitats are similar. We further hypothesized that (2) the trophic structure of oribatid mite communities will change with soil depth with species lower in the food web being more frequent at deeper layers of scree habitats. We also hypothesized that (3) the number of trophic levels in this resource poor habitat will be lower than reported from soils of forests and meadows due to resource scarcity. Finally, we hypothesized that (4) the reproductive mode of oribatid mites in mountain scree is mainly sexual, as resource poor habitats are assumed to be dominated by sexual species.
Material and methods
Study sites
Oribatid mites were sampled in the framework of a long-term study on invertebrate communities in scree habitats and caves in Piatra Craiului National Park, Southern Carpathians, one of the most important karst areas of Romania (Nitzu et al. 2014). Piatra Craiului Massif is a 20 km2 limestone ridge, where more than 500 caves were identified and diverse types of talus and scree slopes, both covered and open, are present (Culver and Pipan 2009). Two types of scree slopes were selected, (1) Cerdacul Stanciului, a mobile limestone scree situated near Stanciului Cave and (2) Marele Grohotis, the largest mobile nude limestone scree accumulation from Piatra Craiului Massif (Fig. 1).
Cerdacul Stanciului and Marele Grohotis are sub-alpine habitats located at 1650 and 1580 m, respectively, and were classified as "calcareous and calcashist screes of the montane to alpine level – Thlaspietea rotundifolii" (Doniță et al. 2005) and are listed in the 8210 habitat types following the Natura 2000 habitat classification. Doniță et al. (2005) included both sites in R6109 type: South-Eastern Carpathian communities of semi-mobile and mobile screes. Mean temperature between April and November 2008 at both scree sites is ca. 16 °C at 50 cm and ca. 10 °C at 75 cm depth (for details see Nae and Băncilă 2017).
Piatra Craiului Massif is a limestone ridge and this type of rock represents 39.5% of the ridge, which favours water infiltration. Therefore, the circulation of underground water plays an important role in the denudation of the relief. Most of the precipitation is infiltrating the rock, resulting in humidity deficiency on the limestone slopes and preventing soil or vegetation development (Constantinescu 2009). This process favours gelifraction, a process that produces rock torrents (which forms through rolling and collapsing limestone), the characteristic geological forms found in Piatra Craiului.
Sampling, extraction and determination
Oribatid mites included in this study were collected using drillings, a special type of pitfall traps adapted for screes (López and Oromi 2010). They consist of PVC tubes, with a pitfall trap inserted at the base. The upper part of the drilling was covered with a plastic lid to prevent debris and rocks from falling inside and the tube had holes drilled at the base, so only animals from that level could enter the trap. We assumed this way of sampling to collect the whole soil oribatid mite community; however, this needs confirmation using other sampling techniques such as heat extraction. Each trap was filled by half with 70% ethanol and an attractant (fermented cheese; Jordana et al. 2020), and was emptied once a month from April to November in 2008 and 2009. The mountain scree habitat was sampled at two depths, 50 and 75 cm.
Oribatid mites were sorted under the stereo microscope and identified to species level with a microscope (Olympus CH2) using the keys of van der Hammen (1952, 1959), Bernini (1978), Pérez-Iñigo (1993, 1997) and Weigmann (2006). The systematic ranking of the species was done after Subías (2004). After identification, the material was preserved in 70% ethanol and stored until stable isotope analysis. The sexing of the species was done based on the list in the Appendix of Maraun et al. (2019).
Stable isotope analysis
Stable isotope ratios of abundant oribatid mite species of the study sites were analysed (see species list in Table 1). Between 1 and 100 individuals were bulked per sample ranging between 1.17 and 10.93 mg of animal tissue.
Food resources of oribatid mite species in mountain scree originate from the epigean environment (Mammola et al. 2016), and therefore we collected surface soil samples at both study sites using a small spade. Both sampling sites have a similar vegetation cover and type of soil. Additionally, we sampled potential food resources of oribatid mites from the study sites, namely roots, mosses and lichens. All samples were dried at 100 °C for 24 h in a desiccator, grounded with mortar and pestle, and subsequently measured for 15N and 13C signatures.
15N/14N and 13C/12C ratios of oribatid mites and surface samples were determined by a coupled system of an elemental analyser (NA1110, Carbo Erba, Milan) and a mass spectrometer (MAT Delta Plus, Finnigan). Isotope signatures are reported using the δ notation with δ15N or δ13C (‰) = (Rsample – Rstandard)/Rstandard × 1000, with Rsample and Rstandard representing the 15N/14N and 13C/12C ratios of the sample and the standard, respectively. Nitrogen in atmospheric air was used as primary standard for 15N, and acetanilide was used for internal calibration.
Statistical analysis
Before statistical analysis, stable isotope data of oribatid mite species and their potential food resources (soil, mosses and lichens) from the two study sites ‘Cerdacul Stanciului’ and ‘Marele Grohotis’ were calibrated to the stable isotope signature of roots used as baseline. The calibrated data were analysed using Discriminant Function Analysis (DFA) with the grouping variable ‘site’ with two levels (‘Cerdacul Stanciului’ and ‘Marele Grohotis’), and the 15N and 13C values of oribatid mites as dependent variables. Subsequently, we used ANOVA to separately analyse 15N and 13C values of the oribatid mite species that occurred at both sites.
Only two oribatid mite species occurred at both sites in sufficient numbers (i.e., at least two replicates per site) for statistical comparison, namely Oribatella foliata and Ceratoppia bipilis. We investigated if their stable isotope signatures differed between sites. We performed a Discriminant Function Analysis (DFA) with the grouping variable ‘sites’ with two levels (‘Cerdacul Stanciului’ and ‘Marele Grohotis’), and the two dependent factors ‘15N’ and ‘13C’. As the analysis was only significant for C. bipilis (for details see below) we subsequently tested (using ANOVA) which of the two factors (‘15N’ and ‘13C’) was responsible for the differences. Second, we performed another DFA with the grouping variable ‘depth’ with two levels (50 and 75 cm) and the two independent factors ‘15N’ and ‘13C’. All statistical analyses were carried out using Statistica v.13.5.0 (Tibco Statistica, Palo Alto, CA, USA; https://docs.tibco.com/products/tibco-statistica-13-5-0).
Results
Twelve oribatid mite species exclusively occurred at the Marele Grohotis site (Table 1), whereas no oribatid mite species occurred exclusively at the Cerdacul Stanciului site; five oribatid mite species occurred at both sites (Ceratoppia bipilis, Chamobates cuspidatus, Oribatella calcarata, Oribatella foliata and Oribatella quadricornuta); however, only two species (C. bipilis and O. foliata) occurred at both sites in sufficient numbers for statistical analyses.
Variations between study sites
Stable isotope signatures (root calibrated data) of the oribatid mite communities from ‘Cerdacul Stanciului’ and ‘Marele Grohotis’ were significantly different (DFA: Wilks’ λ = 0.81; approx. F2,53 = 6.01, p = 0.004) which was due to differences in 13C (ANOVA: F1,54 = 10.60, p = 0.002) but not in 15N values (F1,54 = 1.80, p = 0.18). However, only two species (O. foliata and C. bipilis) occurred in sufficient numbers to allow at least two stable isotope measurements per site. 15N and 13C values of O. foliata did not differ significantly between ‘Cerdacul Stanciului’ and ‘Marele Grohotis’ (DFA: Wilks’ λ = 0.14; approx. F2,2 = 5.94, p = 0.14), whereas those of C. bipilis differed significantly between the two sites (DFA: Wilks’ λ = 0.13; approx. F2,7 = 21.93, p < 0.001), which was due to differences in 13C (ANOVA: F1,8 = 40.9, p < 0.001) but not in 15N values (F1,8 = 0.50, p = 0.50).
Variations with soil depth
The depth where the samples were taken from (50 and 75 cm) did not significantly affect the stable isotope signatures of oribatid mite species (root calibrated data) at the two study sites (DFA: Wilks’ λ = 0.97; approx. F2,53 = 0.79, p = 0.46).
Number of trophic levels
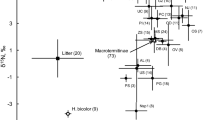
15N data of oribatid mite species pooled for study sites indicated that they comprised three trophic levels (Fig. 2). The lowest trophic level (lichen feeders) included only one species, Carabodes labyrinthicus. Ten species were grouped as primary decomposers including C. marginatus, D. acutus, E. globosus, E. acromios, E. torulosus, H. gibba, L. coracinus, O. calcarata, P. willmanni and X. tegeocranus. Six species were grouped as secondary decomposers/fungal feeders including C. cuspidatus, C. bipilis, C. areolatus, O. foliata, O. quadricornuta and O. tibialis. Species of the same genus often were ascribed to different trophic levels, e.g., in the three species of the genus Carabodes, with C. marginatus grouped as primary decomposer, C. areolatus as secondary decomposer and C. labyrinthicus as lichen feeder. Similarly, in Oribatella species, O. calcarata was grouped as primary decomposer, whereas O. quadricornuta was grouped as secondary decomposer. By contrast, both Eupelops species (E. torulosus and E. acromios) were grouped as primary decomposers.
Stable isotope signatures (δ13C [‰] and δ15N [‰]) (means ± SD) of oribatid mite species from the studied mountain scree habitats in the Romanian Carpathians (data pooled for the two study sites, Cerdacul Stanciului and Marele Grohotis, and the two sampling depths, 50 and 75 cm). Signatures of roots, mosses and lichens are given for comparison
Reproductive mode
All species recorded at the two study sites were sexual suggesting that parthenogenetic species do not exist or are very rare.
Discussion
Trophic structure
Findings of our study indicated that stable isotope signatures between oribatid mite communities from the two mountain scree sites (Cerdacul Stanciului, Marele Grohotis) differed only little, and that the (limited) differences between the two communities likely were due to different species occurring at the two sites rather than differences in trophic niches between the same species. In fact, the isotope signatures of the species that occurred at both sites in sufficient numbers to allow stable isotope analysis were similar. Only one species, C. bipilis, had higher 13C signatures in Cerdacul Stanciului than in Marele Grohotis. This suggests that the food resources used by oribatid mite communities at the two sites are similar.
The trophic structure of oribatid mite communities at the two study sites was not significantly affected by the depth of the scree they were sampled (50 and 75 cm). This indicates that the composition of food resources that are consumed by oribatid mite species changes little with depth, at least in deep scree habitats. Presumably, food resources originate in large from the plants (including cryptogams) growing at the surface or from soil covering scree slopes. Little variation in trophic niches with depth in oribatid mites in scree habitats is in agreement with other studies where stable isotope values of oribatid mites also have been shown to vary little with soil depth (Scheu and Falca 2000; Potapov et al. 2019).
Oribatid mite communities at the two studied mountain scree sites spanned three trophic levels and included lichen feeders, primary decomposers and secondary decomposers. However, lichen feeders were only represented by one species (which only occurred at low density at one site, Marele Grohotis); based on detritus as basal resource the communities only included two trophic levels, i.e., primary and secondary decomposers. Compared to other habitats this is rather low; for oribatid mites in temperate and tropical forest, in meadows and in salt marshes the number of trophic levels (as indicated by δ15N values) typically is four (Illig et al. 2005; Fischer et al. 2010; Perdomo et al. 2012; Magilton et al. 2019). Only two studies on oribatid mites in dead wood (Bluhm et al. 2015) and in sporocarps of Fomitopsis (Maraun et al. 2014) reported only three trophic levels. This indicates that the resource conditions in mountain scree are rather poor restricting energy supply of higher trophic levels (Brown et al. 2004).
Reproductive mode
All oribatid mite species recorded were reproducing sexually, whereas parthenogenetic oribatid mite taxa dominating in many terrestrial habitats, such as Nothrina, Brachychthoniidae, Suctobelbidae and species of the genus Tectocepheus (Maraun and Scheu 2000), were missing. As indicated by low density and few trophic levels, mountain scree habitats are resource-poor, and this might be related to the dominance of sexual species as oribatid mites in other resource-poor habitats, such as tropical soils, dead wood, fungal sporocarps, salt marshes and caves, also are dominated by sexual species (Scheu and Drossel 2007; Maraun et al. 2019). By contrast, in habitats with ample resources and high densities of oribatid mites, such as boreal and temperate forests, parthenogenetic species dominate (Maraun et al. 2012).
Species composition
All 17 species collected at the two study sites were Brachypylina. Many of the species are known from trees, rocks and caves. Carabodes labyrinthicus exclusively lives on lichens (Reeves 1988; Erdmann et al. 2007; Fischer et al. 2010) and stable isotope values suggest that this species also feeds on lichens. Ten species were assigned to primary decomposers (C. marginatus, D. acutus, E. globosus, E. acromios, E. torulosus, H. gibba, L. coracinus, O. calcarata, P. willmanni, X. tegeocranus) known to feed on detritus or fungi (Schneider et al. 2004; Norton and Behan-Pelletier 2009; Fischer et al. 2010; Maraun et al. 2011). Their presence indicates that in the scree habitat small amounts of organic material are present. Six species were assigned to secondary decomposers (C. areolatus, C. bipilis, C. cuspidatus, O. foliata, O. quadricornuta, O. tibialis). These species are also often found in forest soils but some of them are also regularly found in caves, namely C. areolatus, C. bipilis and C. cuspidatus (Bruckner 1995). Their high stable isotope signatures and their occurrence in resource-poor habitats such as caves indicates that these species are able to cope with extremely resource poor conditions and feed on the few resources present in mountain scree or caves (Mock et al. 2005). Very likely, their main food resources are fungi (Hågvar and Steen 2013; Hågvar et al. 2014). This is also supported by other stable isotope studies which found these species to be mainly secondary decomposers feeding on fungi (Fischer et al. 2010; Maraun et al. 2011; Erdmann et al. 2012; Bluhm et al. 2015; Maaß et al. 2015). Notably, the three Oribatella species (O. quadricornuta, O. foliata and O. calcarata) as well as the three species of the genus Carabodes (C. areolatus, C. marginatus and C. labyrinthicus) were ascribed to different trophic levels indicating that different species within genera may consume different resources.
Several oribatid mite species (e.g., O. quadricornuta, O. calcarata, Parachipteria willmanni) had high variation in 15N signatures indicating that their trophic niche is rather broad and that they feed on a wide range of resources. For example, P. willmanni occurs in forest soils, but also in peat bogs and in mountain scree (Wood and Lawton 1973; Lehmitz and Maraun 2016). This species may feed mainly on fungi, but it may also occasionally ingest resources such as mosses, which would explain their rather low 15N signatures and their high variation.
δ13C values were high for L. coracinus, H. gibba, X. tegeocranus and D. acutus. This was known before for Liacarus and Carabodes species (Maraun et al. 2011), and is likely due to the fact that these species incorporate calcium carbonate to harden their exoskeleton (Norton and Behan-Pelletier 1991). As the standard for 13C measurement (Pee Dee Belemnite limestone) with a 13C signature of zero reflects, inorganic carbonates are much less depleted in 13C than organic matter.
Overall, results of our study show that mountain scree habitats are colonized by specific oribatid mite communities comprising few species of derived taxa that reproduce sexually. Including lichen feeders, the species spanned three trophic levels, but predatory species were lacking suggesting limited resource availability for higher trophic levels. Low densities, sexual reproduction and few trophic levels all presumably reflect resource poor conditions in mountain scree habitats.
References
Albers D, Schaefer M, Scheu S (2006) Incorporation of plant carbon into the soil animal food web of an arable system. Ecology 87:235–245. https://doi.org/10.1890/04-1728
Badejo MA, Akinwole PO (2006) Microenvironmental preferences of oribatid mite species on the floor of atropical rainforest. Exp Appl Acarol 40:145–156. https://doi.org/10.1007/s10493-006-9029-y
Bernini F (1978) Notulae oribatologicae XX. II genere Oribatella in Italia (Acarida, Oribatida). Redia 61:503–538
Bluhm C, Scheu S, Maraun M (2015) Oribatid mite communities on the bark of dead wood vary with log type, surrounding forest and regional factors. Appl Soil Ecol 89:102–112. https://doi.org/10.1016/j.apsoil.2015.01.013
Bluhm C, Scheu S, Maraun M (2016) Temporal fluctuations in oribatid mites indicate that density-independent factors favour parthenogenetic reproduction. Exp App Acarol 68:387–407. https://doi.org/10.1007/s10493-015-0001-6
Brown JH, Gillooly JF, Allen AP, van Savage M, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789. https://doi.org/10.1890/03-9000
Bruckner A (1995) Cave-dwelling oribatid mites (Acarina, Cryptostigmata) from East Austria. Verh Zool-Bot Ges Österreich 132:81–107
Caruso T, Trokhymets V, Bargagli R, Convey P (2013) Biotic interactions as a structuring force in soil communities: evidence from the microarthropods of an Antarctic moss model system. Oecologia 172:495–503. https://doi.org/10.1007/s00442-012-2503-9
Caruso T, Schaefer I, Monson F, Keith AM (2019) Oribatid mites show how climate and latitudinal gradients in organic matter can drive large-scale biodiversity patterns of soil communities. J Biogeogr 46:611–620. https://doi.org/10.1111/jbi.13501
Constantinescu T (2009) Piatra Craiului - Studiu geomorfologic. Universitara Bucuresti, Bucharest, p 163
Culver DC, Pipan T (2009) Superficial subterranean habitats—gateway to the subterranean realm? Cave Karst Sci 35:5–12
Culver DC, Pipan T (2019) The biology of caves and other subterranean habitats. Oxford University Press, Oxford
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. https://doi.org/10.1016/0016-7037(81)90244-1
Doniță N, Popescu A, Comanescu M, Mihailescu S, Biris IA (2005) Habitatele din România. Editura Tehnică Silvică, București, p 496
Erdmann G, Otte V, Langel R, Scheu S, Maraun M (2007) The trophic structure of bark-living oribatid mite communities analysed with stable isotopes (15N, 13C) indicates strong niche differentiation. Exp Appl Acarol 41:1–10. https://doi.org/10.1007/s10493-007-9060-7
Erdmann G, Scheu S, Maraun M (2012) Regional factors rather than forest type drive the community structure of soil living oribatid mites (Acari, Oribatida). Exp Appl Acarol 57:157–169. https://doi.org/10.1007/s10493-012-9546-9
Fischer BM, Schatz H, Maraun M (2010) Community structure, trophic position and reproductive mode of soil and bark-living oribatid mites in an alpine grassland ecosystem. Exp Appl Acarol 52:221–237. https://doi.org/10.1007/s10493-010-9366-8
Hågvar S, Steen R (2013) Succession of beetles (genus Cis) and oribatid mites (genus Carabodes) in dead sporocarps of the red-banded polypore fungus Fomitopsis pinicola. Scand J For Res 28:436–444. https://doi.org/10.1080/02827581.2012.755562
Hågvar S, Amundsen T, Økland B (2014) Mites of the genus Carabodes (Acari, Oribatida) in Norwegian coniferous forests: occurrence in different soils, vegetation types and polypore hosts. Scand J For Res 29:629–638. https://doi.org/10.1080/02827581.2014.965195
Illig J, Norton RA, Langel R, Scheu S, Maraun M (2005) Where are the decomposers? Uncovering the soil food web of a tropical montane rain forest in Southern Ecuador using stable isotopes (15N). J Trop Ecol 21:589–593. https://doi.org/10.1017/S0266467405002646P
Jiménez-Valverde A, Gilgado JD, Sendra A, Pérez-Suárez G, Herrero-Borgoñón JJ, Ortuño VM (2015) Exceptional invertebrate diversity in a scree slope in Eastern Spain. J Insect Conserv 19:713–728. https://doi.org/10.1007/s10841-015-9794-1
Jordana R, Baquero E, Ledesma E, Sendra A, Ortuño VM (2020) Poduromorpha (Collembola) from a sampling in the mesovoid shallow substratum of the Sierra de Guadarrama National Park (Madrid and Segovia, Spain): Taxonomy and biogeography. Zool Anz 285:81–96. https://doi.org/10.1016/j.jcz.2020.02.001
Juberthie C (1983) Le milieu souterrain: Etendue et composition. Mem Biospeol 10:17–65
Juberthie C, Delay B, Bouillon M (1980) Extension du milieu souterrain en zone calcaire: description d’un nouveau milieu et de son peuplement par les Coleopteres troglobies. Mem Biospeol 7:19–52
Lehmitz R, Maraun M (2016) Small-scale spatial heterogeneity of stable isotopes signatures (δ15N, δ13C) in Sphagnum sp. transfers to all trophic levels in oribatid mites. Soil Biol Biochem 100:242–251. https://doi.org/10.1016/j.soilbio.2016.06.005
Lindberg N, Bengtsson J (2005) Population responses of oribatid mites and collembolans after drought. Appl Soil Ecol 28:163–174. https://doi.org/10.1016/j.apsoil.2004.07.003
López H, Oromi P (2010) A pitfall trap for sampling the mesovoid shallow substratum (MSS) fauna. Speleobiol Notes 2:7–11
Maaß S, Maraun M, Scheu S, Rillig MC, Caruso T (2015) Environmental filtering vs. resource-based niche partitioning in diverse soil animal assemblages. Soil Biol Biochem 85:145–152. https://doi.org/10.1016/j.soilbio.2015.03.005
Magilton M, Maraun M, Emmerson M, Caruso T (2019) Oribatid mites reveal that competition for resources and trophic structure combine to regulate the assembly of diverse soil animal communities. Ecol Evol 9:8320–8330. https://doi.org/10.1002/ece3.5409
Mammola S, Giachino PM, Piano E, Jones A, Barberis M, Badino G, Isaia M (2016) Ecology and sampling techniques of an understudied subterranean habitat: the Milieu Souterrain Superficiel (MSS). Sci Nat 103:88. https://doi.org/10.1007/s00114-016-1413-9
Maraun M, Scheu S (2000) The structure of oribatid mite communities (Acari, Oribatida): patterns, mechanisms and implications for future research. Ecography 23:374–383. https://doi.org/10.1111/j.1600-0587.2000.tb00294.x
Maraun M, Salamon JA, Schneider K, Schaefer M, Scheu S (2003) Oribatid mite and collembolan diversity, density and community structure in a moder beech forest (Fagus sylvatica): effects of mechanical disturbances. Soil Biol Biochem 35:1387–1394. https://doi.org/10.1016/S0038-0717(03)00218-9
Maraun M, Schatz H, Scheu S (2007) Awesome or ordinary? Global diversity patterns of oribatid mites. Ecography 30:209–216. https://doi.org/10.1111/j.0906-7590.2007.04994.x
Maraun M, Erdmann G, Fischer BM, Pollierer MM, Norton RA, Schneider K, Scheu S (2011) Stable isotopes revisited: their use and limits for oribatid mite trophic ecology. Soil Biol Biochem 43:877–882. https://doi.org/10.1016/j.soilbio.2011.01.003
Maraun M, Norton RA, Ehnes R, Scheu S, Erdmann G (2012) Positive correlation of density and parthenogenetic reproduction in oribatid mites supports the “structured resource theory of sexual reproduction.” Evol Ecol Res 14:311–323
Maraun M, Augustin D, Müller J, Bässler C, Scheu S (2014) Changes in the community composition and trophic structure of microarthropods in sporocarps of the wood decaying fungus Fomitopsis pinicola along an altitudinal gradient. Appl Soil Ecol 84:16–23. https://doi.org/10.1016/j.apsoil.2014.06.004
Maraun M, Caruso T, Hense J, Lehmitz R, Mumladze L, Murvanidze M, Nae I, Schulz J, Seniczak A, Scheu S (2019) Parthenogenetic vs. sexual reproduction in oribatid mite communities. Ecol Evol 9:7324–7332. https://doi.org/10.1002/ece3.5303
Martinez del Rio C, Wolf N, Carleton SA, Gannes LZ (2009) Isotopic ecology ten years after a call for more laboratory experiments. Biol Rev 84:91–111. https://doi.org/10.1111/j.1469-185X.2008.00064.x
Melguizo-Ruiz N, Jiménez-Navarro G, Zieger S, Maraun M, Scheu S, Moya-Laraño J (2017) Complex effects of precipitation and basal resources on the trophic ecology of soil oribatid mites: implications for stable isotope analysis. Eur J Soil Biol 82:98–107. https://doi.org/10.1016/j.ejsobi.2017.08.008
Mock A, Ľuptáčik P, Fenda P, Svatoň J, Országh I, Krumpál M (2005) Terrestrial arthropods inhabiting caves near Veľký Folkmar (Čierna hora Mts., Slovakia). In: Tajovský K, Schlaghamerský J, Pižl V (eds) Contributions to soil zoology in central Europe I. ISB ASCR, České Budějovice, pp 95–101
Mumladze L, Murvanidze M, Maraun M, Salakaia M (2015) Oribatid mite communities along an elevational gradient in Sairme gorge (Caucasus). Exp Appl Acarol 66:41–51. https://doi.org/10.1007/s10493-015-9893-4
Nae I, Băncilă IR (2017) Mesovoid shallow substratum as a biodiversity hotspot for conservation priorities: analysis of oribatid mite (Acari: Oribatida) fauna. Acarologia 57:855–868. https://doi.org/10.24349/acarologia/20174202
Nitzu E, Nae A, Băncilă R, Popa I, Giurginca A, Plăiaşu R (2014) Scree habitats: ecological function, species conservation and spatial-temporal variation in the arthropod community. Syst Biodivers 12:65–75. https://doi.org/10.1080/14772000.2013.878766
Norton RA, Behan-Pelletier VM (1991) Calcium carbonate and calcium oxalate as cuticular hardening agents in oribatid mites (Acari: Oribatida). Can J Zool 69:1504–1511. https://doi.org/10.1139/z91-210
Norton RA, Behan-Pelletier VM (2009) Oribatida. In: Krantz GW, Walter DE (eds) A manual of acarology, 3rd edn. Texas Tech University Press, Lubbock, pp 421–564
Norton RA, Palmer S (1991) The distribution, mechanisms and evolutionary significance of parthenogenesis in oribatid mites. In: Schuster R, Murphy PW (eds) The Acari: reproduction, development and life-history strategies. Chapman and Hall, London
Pérez-Iñigo C (1993) Acari: Oribatei, Poronota. In: Ramos MA et al. (ed). Fauna Ibérica, vol 3, Museo Nacional de Ciencias Naturales, Madrid
Pérez-Iñigo C (1997) Acari, Oribatei, Gymnonota I. In: Ramos MA et al (eds) Fauna Ibérica, vol 9. Museo Nacional de Ciencias Naturales, Madrid
Perdomo G, Evans A, Maraun M, Sunnucks P, Thompson R (2012) Mouthpart morphology and trophic position of microarthropods from soils and mosses are strongly correlated. Soil Biol Biochem 53:56–63. https://doi.org/10.1016/j.soilbio.2012.05.002
Pollierer MM, Langel R, Scheu S, Maraun M (2009) Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotopes (15N/14N and 13C/12C). Soil Biol Biochem 41:1221–1226. https://doi.org/10.1016/j.soilbio.2009.03.002
Ponsard S, Arditi R (2000) What can stable isotopes (δ15N and δ13C) tell about the food web of soil macroinvertebrates? Ecology 81:852–864. https://doi.org/10.1890/0012-9658(2000)081[0852:WCSINA]2.0.CO;2
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Potapov AM, Tiunov AV, Scheu S (2019) Uncovering trophic positions and food resources of soil animals using bulk natural stable isotope composition. Biol Rev 94:37–59. https://doi.org/10.1111/brv.12434
Reeves RM (1988) Distribution and habitat comparison for Carabodes collected from conifer branches with description of brevis Banks and higginsi n. sp. (Acari: Oribatida: Carabodidae). Proc Entomol Soc Wash 90:373–392
Schatz H, Behan-Pelletier VM (2008) Global diversity of oribatids (Oribatida: Acari: Arachnida). Hydrobiol 595:323–328. https://doi.org/10.1007/s10750-007-9027-z
Scheu S, Drossel B (2007) Sexual reproduction prevails in a world of structured resources in short supply. Proc R Soc B 274:1225–1231. https://doi.org/10.1098/rspb.2007.0040
Scheu S, Falca M (2000) The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: stable isotope analysis of macro- and a mesofauna-dominated community. Oecologia 123:285–286
Schneider K, Migge S, Norton RA, Scheu S, Langel R, Reineking A, Maraun M (2004) Trophic niche differentiation in soil microarthropods (Oribatida, Acari): evidence from stable isotopes ratios (15N/14N). Soil Biol Biochem 36:1769–1774. https://doi.org/10.1016/j.soilbio.2004.04.033
Skubała P, Dethier M, Madej G, Solarz K, Mąkol J, Kaźmierski A (2013) How many mite species dwell in subterranean habitats? A survey of Acari in Belgium. Zool Anz 252:307–318. https://doi.org/10.1016/j.jcz.2012.09.001
Subías LS (2004) Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Graellsia 60:3–305. https://doi.org/10.3989/graellsia.2004.v60.iExtra.218
Tiunov AV (2007) Stable isotopes of carbon and nitrogen in soil ecological studies. Biol Bull 34:395–407. https://doi.org/10.1134/S1062359007040127
van der Hammen L (1952) The oribatei (Acari) of the Netherlands. Zool Verh 17:1–139
van der Hammen L (1959) Berlese’s primitive oribatid mites. Zool Verh 40:1–93
Walter DE, Proctor HC (1999) Mites: ecology evolution and behaviour. University of NSWPress, Sydney
Weigmann G (2006) Hornmilben (oribatida). Die Tierwelt Deutschlands, 76. Teil Goecke and Evers, Keltern, p 520
Wood TG, Lawton JH (1973) Experimental studies on the respiratory rates of mites (Acari) from beech-woodland leaf litter. Oecologia 12:169–191. https://doi.org/10.1007/BF00345516
Acknowledgements
The authors would like to thank Dr. Ioana Meleg from Emil Racovitza Institute of Speleology and R.A. Norton for valuable comments on the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nae, I., Nae, A., Scheu, S. et al. Oribatid mite communities in mountain scree: stable isotopes (15N, 13C) reveal three trophic levels of exclusively sexual species. Exp Appl Acarol 83, 375–386 (2021). https://doi.org/10.1007/s10493-021-00597-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00597-4