Abstract
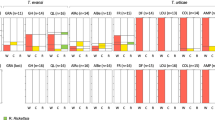
A phage density model of cytoplasmic incompatibility (CI), which means lytic phages reduce bacterial density associated with CI, significantly enhances our understanding of the tripartite associations among bacteriophage WO, Wolbachia and host. However, WO may alternate between lytic and lysogenic life cycles or change phage production under certain conditions including temperature, host age and host species background. Here, extreme temperatures can induce an alteration in the life cycle of WO and change the tripartite associations among WO, Wolbachia and CI. Based on the accumulation of the WO load, WO can transform into the lytic life cycle with increasing age. These findings confirmed that the environment plays an important role in the associations among WO, Wolbachia and host.





Similar content being viewed by others
References
Ahantarig A, Trinachartvanit W, Chauvatcharin N, Kittayapong P, Baimai V (2008) Wolbachia and bacteriophage WO-B density of Wolbachia A-Infected Aedes albopictus mosquito. Folia Microbiol 53:547–550
Bandi C, Anderson TJC, Genchi C, Blaxter ML (1998) Phylogeny of Wolbachia in filarial nematodes. Proc R Soc Lond Ser B 265:2407–2413
Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M (2002) High Wolbachia density in insecticide-resistant mosquitoes. Proc Biol Sci 269:1413–1416
Bordenstein SR, Wernegreen JJ (2004) Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol 21:1981–1991
Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ (2006) The tripartite associations between Bacteriophage, Wolbachia, and Arthropods. PLoS Pathog 2:e43
Braig HR, Zhou W, Dobson S, O’Neill SL (1998) Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180:2373–2378
Chafee ME, Funk DJ, Harrison RG, Bordenstein SR (2010) Lateral transfer in obligate intracellular bacteria (Wolbachia): verification from natural populations. Mol Biol Evol 27:501–505
Chauvatcharin N, Ahantarig A, Baimai V, Kittayapong P (2006) Bacteriophage WO-B and Wolbachia in natural mosquito hosts: infection incidence, transmission mode and relative density. Mol Ecol 15:2451–2461
Clarke GM (1997) The genetic basis of developmental stability III. Haplo-Diploidy: are males more unstable than females? Evolution 51:2021–2028
Degnan PH, Moran NA (2008) Diverse phage-encoded toxins in a protective insect endosymbiont. Appl Environ Microbiol 74:6782–6791
Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, O’Neill SL (1999) Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol 29:153–160
Duron O, Fort P, Weill M (2006) Hypervariable prophage WO sequences describe an unexpected high number of Wolbachia variants in the mosquito Culex pipiens. Proc R Soc B 273:495–502
Duron O, Fort P, Weill M (2007) Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity 98:368–374
Echaubard P, Duron O, Agnew P, Sidobre C, Noel V, Weill M, Michalakis Y (2010) Rapid evolution of Wolbachia density in insecticide resistant Culex pipiens. Heredity 104:15–19
Fujii Y, Kubo T, Ishikawa H, Sasaki T (2004) Isolation and characterization of the bacteriophage WO from Wolbachia, an arthropod endosymbiont. Biochem Biophys Res Commun 317:1183–1188
Gavotte L, Vavre F, Henri H, Ravallec M, Stouthamer R, Boulétreau M (2004) Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol Biol 13:147–153
Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, Bouletreau M, Vavre F (2007) A survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia. Mol Biol Evol 24:427–435
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow AJ, Werren H (2008) How many species are infected with Wolbachia?—a statistical analysis of current data. FEMS Microbiol Lett 281:215–220
Hurst GDD, Jiggins FM, Schulenburg JHG, Bertrand D, West SA, Goriacheva II, Zakharov IA, Werren JH, Stouthamer R, Majerus MEN (1999) Male killing Wolbachia in two species of insects. Proc R Soc Lond Ser B 266:735–740
Jamnongluck W, Kittayapong P, Baisley KJ, O’Neill SL (2000) Wolbachia infection and expression of cytoplasmic incompatibility in Armigeres subalbatus (Diptera: Culicidea). J Med Entomol 37:53–57
Kent BN, Bordenstein SR (2010) Phage WO of Wolbachia: lambda of the endosymbiont world. Trends Microbiol 18:173–181
Masui S, Sasaki T, Ishikawa H (2000) Genes for the type IV secretion system in an intracellular symbiont, Wolbachia, a causative agent of various sexual alterations in arthropods. J Bacteriol 182:6529–6531
Miao EA, Miller SI (1999) Bacteriophages in the evolution of pathogen–host interactions. Proc Natl Acad Sci USA 96:9452–9454
Noda H, Koizumi Y, Zhang Q, Deng K (2001) Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem Mol Biol 31:727–737
Oliver KM, Degnan PH, Hunter MS, Moran NA (2009) Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325:992–994
Opijne TV, Breeuwer JAJ (1999) High temperatures eliminate Wolbachia, a cytoplasmic incompatibility inducing endosymbiont, from the two-spotted spider mite. Exp Appl Acarol 23:871–881
Reynolds KT, Hoffmann AA (2002) Male age, host effects and the weak of expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by the maternally inherited Wolbachia. Genet Res 80:79–87
Riegler M, O’Neill SL (2006) The genus Wolbachia. Prokaryotes 5:547–561
Ros VID, Fleming VM, Feil EJ, Breeuwer JA (2009) How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl Environ Microbiol 75:1036–1043
Sasaki T, Ishikawa H (1999) Wolbachia infections and cytoplasmic incompatibility in the almond moth and the Mediterranean flour moth. Zool Sci 16:739–744
Stouthamer R, Breeuwer JA, Hurst GD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Tanaka K, Furukawa S, Nikoh N, Sasaki T, Fukatsu T (2009) Complete WO phage sequences reveal their dynamic evolutionary trajectories and putative functional elements required for integration into the Wolbachia genome. Appl Environ Microbiol 75:5676–5686
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tortosa P, Charlat S, Labbe P, Dehecq JS, Barre H, Weill M (2010) Wolbachia age-sex-specific density in aedes albopictus: a host evolutionary response to cytoplasmic incompatibility? PLoS One 5:e9700
Turelli M, Hoffmann AA (1995) Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319–1338
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42:587–609
Werren JH, Zhang W, Guo LR (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc Lond Ser B 261:55–71
Wiwatanaratanabutr S, Kittayapong P (2006) Effects of temephos and temperature on Wolbachia load and life history traits of Aedes albopictus. Med Vet Entomol 20:300–307
Wiwatanaratanabutr I, Kittayapong P (2009) Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J Invertebr Pathol 102:220–224
Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I, Markakis G, Brissac T, Merçot H, Bourtzis K (2008) Multiple Rescue Factors within a Wolbachia Strain. Genetics 178:2145–2160
Zhou WG, Rousset F, O’Neill S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B 265:509–515
Zhou YS, Pu CS, Meng W, Yang SX (2003) Influence of temperature on development and population dynamics of Tetranychus urticae Koch. J Shenyang Agric Univ 34:99–102
Acknowledgments
We would like to thank Jingtao Sun, Mingzhi Yu and Rongrong Xie of the Department of Entomology, Nanjing Agricultural University (NJAU) for help with the collection of spider mites. We are also grateful to Lili Zhou of the Department of Entomology, NJAU for her technical help with experiments and to Zhiyuan Ji of NJAU for his critical reading of the early draft. This study was supported in part by a Special Fund for Agro-Scientific Research in the Public Interest (No. 201103020) from the Ministry of Agriculture of China and a Grant-in-Aid for Scientific Research (No. 31172131 and No. 30871635) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, MH., Zhang, KJ. & Hong, XY. Tripartite associations among bacteriophage WO, Wolbachia, and host affected by temperature and age in Tetranychus urticae . Exp Appl Acarol 58, 207–220 (2012). https://doi.org/10.1007/s10493-012-9578-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9578-1