Abstract
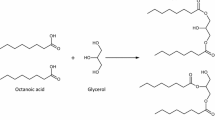
Liacarus subterraneus is a large, soil-dwelling oribatid mite species that possesses a conspicuously shiny, clean and not wettable cuticular surface. The exocrine cuticular chemistry of this species was investigated by means of gas chromatography–mass spectrometry. Besides a fraction of hydrocarbons and a terpene, hexane extracts of whole mite bodies exhibited free carboxylic acids and their glycerides as main components. The compounds were arranged in three distinct extract profiles. Based on data from individual extracts, (1) the majority (more than 3/4) of specimens showed large amounts of 1,2-dioctanoyl-glycerol (and three other related esters) but no (or only traces of) free carboxylic acids. (2) In about 1/8 of extracts, free acids (mainly octanoic (caprylic) acid) and glycerides were detected. This second type of profile highly varied with respect to the relative abundance of acids and esters. (3) The third profile (in about 7% of specimens) exclusively exhibited free acids and no (or only traces of) glycerides. In addition, a few extracts exhibited no components at all. The extract compounds most likely originate from the lipid layer of the cerotegument of L. subterraneus. The cuticle of individuals that possessed extractable cerotegumental compounds (profile I, II, III) exhibited strong water repellent properties, while the cuticle of individuals that possessed no components in their extract did not. After hexane extraction, water repellent properties got lost. The distinct extract profiles detected most likely portray the stepwise generation of an anti-wetting, exocrine surface lipid layer of glycerides: If this layer is lost, fatty acids may be discharged again (profile III) and may subsequently esterify (profile II) to larger and more stable esters (diacyl-glycerols), eventually building up the “raincoat” (mainly profile I) of L. subterraneus.


Similar content being viewed by others
References
Alberti G, Coons LB (1999) Mites. In: Harrison FW, Foelix RF (eds) Microscopic anatomy of invertebrates, vol. 8C. Chelicerate Arthropoda. Wiley–Liss, New York, pp 515–1265
Alberti G, Storch V, Renner H (1981) Über den feinstrukturellen Aufbau der Milbencuticula. Zool Jb Anat Abt 105:183–236
Alberti G, Norton RA, Adis J, Fernandez NA, Kratzmann M, Moreno A, Ribiero E, Weigmann G, Woas S (1997) Porose integumental organs of oribatid mites (Acari, Oribatida). 2. Fine structure. Zool Stuttg 146:33–114
Attygalle AB, Wu X, Ruzicka J, Rao S, Garcia S, Herath K, Meinwald J, Maddison DR, Will KW (2004) Defensive chemicals of two species of Trachypachus Motschulski. J Chem Ecol 30:577–588. doi:10.1023/B:JOEC.0000018630.79922.94
Barthlott W, Neinhuis C (1997) Purity of sacred lotus, or escape from contamination in biological surfaces. Planta 202:1–8. doi:10.1007/s004250050096
Barthlott W, Neinhuis C (1998) Lotus-Effekt und Autolack: Die Selbstreinigungsfähigkeit mikrostrukturierter Oberflächen. Biol Unserer Zeit 28:314–321. doi:10.1002/biuz.960280507
Cardé RT, Millar JG (2004) Advances in insect chemical ecology. Cambridge University Press, Cambridge, p 341
Eisner T, Meinwald J, Monro A, Ghent R (1961) Defense mechanisms of arthropods. I. The composition and function of the sray of the whipscorpion, Mastigoproctus giganteus. J Insect Physiol 6:272–298. doi:10.1016/0022-1910(61)90054-3
Eisner T, Eisner M, Siegler M (2005) Secret weapons. Harvard University Press, Harvard, p 372
Evans GO (1992) Priciples of acarology. CAB International, Wallingford, p 563
Filshie BK (1982) Fine structure of the cuticle of insects and other arthropods. In: King RC, Akai H (eds) Insect ultrastructure. Plenum Press, New York, pp 281–312
Fortunato A, Maile R, Turillazzi S, Morgan ED, Moneti G, Jones GR, Pieraccini G (2001) Defensive role of secretion of ectal mandibular glands of the wasp Polistes dominulus. J Chem Ecol 27:569–579. doi:10.1023/A:1010393006831
Haupt J, Müller F (2004) New products of defense secretion in South East Asian whip scorpions (Arachnida: Uropygi: Thelyphonida). Zeitschr Naturforsch 59C:579–581
Huwyler S, Grob K, Viscontini M (1975) The trail pheromone of the ant, Lasius fuliginosus. J Insect Physiol 21:299–304. doi:10.1016/0022-1910(75)90025-6
Kabara JJ (1978) Fatty acids and derivatives as antimicrobial agents. In: Kabara JJ (ed) The pharmacological effect of lipids. The American Oil Chemists Society, St Louis, pp 1–14
Koch CL (1844) Deutschlands crustaceen, myriapoden und arachniden, 38(11), Regensburg
Lockey KH (1988) Lipids of the insect cuticle: origin, composition, and function. Comp Biochem Physiol 89B:595–645
Löfstedt C, Hansson BS, Petersson E, Valeur P, Richards A (1994) Pheromonal secretions from glands on the 5th abdominal sternite of hydropsychid and rhyacophilid caddisflies. J Chem Ecol 20:153–170. doi:10.1007/BF02065998
Maraun M, Scheu S (2000) The structure of oribatid mite communities (Acari, Oribatida): patterns, mechanisms and implications for future research. Ecography 23:374–383. doi:10.1034/j.1600-0587.2000.d01-1647.x
Matischek T, Stabentheiner E, Raspotnig G, Krisper G (2004) Elementaranalysen der Cuticula und des Ceroteguments bei Damaeus onustus (C. L. Koch, 1844) und Eupelops torulosus (C. L. Koch, 1836) (Acari: Oribatida). Abh Ber Naturkundemus Gorlitz 76:25–31
Messer C, Dettner K, Schulz S, Francke W (1999) Phenolic compounds in Neanura muscorum (Collembola, Neanuridae) and the role of 1, 3-dimethoxybenzene as an alarm substance. Pedobiologia (Jena) 43:174–182
Nair MKM, Vasudevan P, Hoagland T, Venkitanarayanan K (2004) Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in milk by caprylic acid and monocaprylin. Food Microbiol 21:611–616. doi:10.1016/j.fm.2004.01.003
Nair MKM, Joy J, Vasudevan P, Hinckley L, Hoagland TA, Venkitanarayanan KS (2005) Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J Dairy Sci 88:3488–3495
Neinhuis C, Barthlott W (1997) Characterisation and distribution of water-repellent, self-cleaning plant surfaces. Ann Bot (Lond) 79:667–677. doi:10.1006/anbo.1997.0400
Raspotnig G (2006a) Chemical alarm and defence in the oribatid mite Collohmannia gigantea (Acari: Oribatida). Exp Appl Acarol 39:177–194. doi:10.1007/s10493-006-9015-4
Raspotnig G (2006b) Characterisation of monophyletic oribatid groups by oil gland chemistry—a novel systematic approach in Oribatida (Acari). Abh Ber Naturkundemus Gorlitz 78:31–46
Raspotnig G, Krisper G (1998) Fatty acids as cuticular surface components in oribatid mites (Acari: Oribatida). In: Ebermann E (ed) Arthropod biology: contributions to morphology, ecology and systematics. Biosys Ecol Ser, 14, pp 215–243
Raspotnig G, Krisper G, Fauler G, Leis HJ (2008) Distinctive cuticular hydrocarbon profiles in oribatid mites (Acari: Oribatida). Ann Zool 58:445–452
Sakata T, Shimano S, Kuwahara Y (2003) Chemical ecology of oribatid mites. III. Chemical composition of oil gland exudates from two oribatid mites, Trhypochthoniellus sp. and Trhypochthonius japonicus (Acari: Trhypochthoniidae). Exp Appl Acarol 29:279–291. doi:10.1023/A:1025882214375
Saporito R, Donnelly MA, Norton RA, Garraffo HM, Spande TF, Daly JW (2007) Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc Natl Acad Sci USA 104:8885–8890. doi:10.1073/pnas.0702851104
Schatz H (2002) Die Oribatidenliteratur und die beschriebenen Oribatidenarten (1758–2001)—eine Analyse. Abh Ber Naturkundemus Gorlitz 74:37–45
Schmidt JO, Dani FR, Jones GR, Morgan DE (2000) Chemistry, ontogeny, and role of pygidial gland secretions of the vinegaroon Mastigoproctus giganteus (Arachnida: Uropygi). J Insect Physiol 46:443–450. doi:10.1016/S0022-1910(99)00130-4
Shimano S, Sakata T, Mizutani Y, Kuwahara Y, Aoki J (2002) Geranial: the alarm pheromone in the nymphal stage of the oribatid mite, Nothrus palustris. J Chem Ecol 28:1831–1837. doi:10.1023/A:1020517319363
Smrz J (2006) Microhabitat selection in the simple oribatid community dwelling in epilithic moss cover (Acari: Oribatida). Naturwissenschaften 93:570–576. doi:10.1007/s00114-006-0141-y
Suzuki T, Haga K, Tsutsumi T, Matsuyama S (2004) Analysis of anal secretions of phlaeothripine thrips. J Chem Ecol 30:409–423. doi:10.1023/B:JOEC.0000017985.89897.c3
Takada W, Sakata T, Stimano S, Enami Y, Mori N, Nishida R, Kuwahara Y (2005) Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J Chem Ecol 31:2403–2415. doi:10.1007/s10886-005-7109-9
Tschinkel WR (1975) Comparative studies of the chemical defensive system of tenebrionid beetles. Chemistry of the secretions. J Insect Physiol 21:753–783. doi:10.1016/0022-1910(75)90008-6
Vitzthum H (1940–43) Acarina. In: Bronn’s Klassen und Ordnungen des Tierreichs 5/4/5. Akademische Verlagsgesellschaft, Leipzig, pp 1–1011
Wagner T, Neinhuis C, Barthlott W (1996) Wettability and contaminability of insect wings as a function of their surface sculptures. Acta Zool 77:213–225
Acknowlegdements
This study was supported by the Austrian Science Fund (FWF), project number P18486. We are grateful to Dr. Günther Krisper (Institute of Zoology, Karl-Franzens-University Graz, Austria) for determination of Liacarus subterraneus and for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raspotnig, G., Leis, HJ. Wearing a raincoat: exocrine secretions contain anti-wetting agents in the oribatid mite, Liacarus subterraneus (Acari: Oribatida). Exp Appl Acarol 47, 179–190 (2009). https://doi.org/10.1007/s10493-008-9212-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-008-9212-4