Abstract
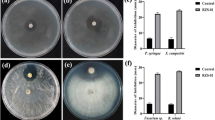
Plants have their own defense mechanisms such as induced systemic resistance (ISR) and systemic-acquired resistance. Bacillus spp. are familiar biocontrol agents that trigger ISR against various phytopathogens by eliciting various metabolites and producing defense enzyme in the host plant. In this study, B. paralicheniformis (strain EAL) was isolated from the medicinal plant Enicostema axillare. Butanol extract of B. paralicheniformis showed potential antagonism against Fusarium oxysporum compared to control well (sterile distilled water) A liquid chromatography mass spectrometry analysis showed 80 different compounds. Among the 80 compounds, we selected citrulline, carnitine, and indole-3-ethanol based on mass-to-charge ratio, database difference, and resolution of mass spectrum. The synthetic form of the above compounds showed biocontrol activity against F. oxysporum under in vitro condition in combination, not as individual compounds. However, the PCR amplification of 11 antimicrobial peptide genes showed that none of the genes amplified in the strain. B. paralicheniformis inoculation challenged with F. oxysporum on tomato plants enhanced production of defense enzymes such as peroxidase (POD), superoxide dismutase (SOD), phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), and proline compared to control plants (without inoculation of B. paralicheniformis) at significant level (p < 0.005). Stem of tomato plants expressed higher POD (2.2-fold), SOD (2.2-fold), PPO (1.9-fold), and PAL (1.3-fold) contents followed by the leaf and root. Elevated proline accumulation was observed in the leaf (1.8-fold) of tomato plants. Thus, results clearly showed potentiality of B. paralicheniformis (EAL) in activation of antioxidant defense enzyme against F. oxysporum-infected tomato plants and prevention of oxidative damage though hydroxyl radicals scavenging activities that suppress the occurrence of wilt diseases.





Similar content being viewed by others
References
Abo-Elyousr KA, Hashem AEH, Ali EH (2009) Integrated control of cotton root rot disease by mixing fungal biocontrol agents and resistance inducers. Crop Prot 28:295–301
Akashi K, Miyake C, Yokota A (2001) Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett 508:438–442
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Anand T, Chandrasekaran A, Kuttalam S, Raguchander T, Prakasam V, Samiyappan R (2007) Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J Plant Interact 2:233–244
Anthony KK, George DS, Baldev Singh HK, Fung SM, Santhirasegaram V, Razal Z, Somasundram C (2017) Reactive oxygen species activity and antioxidant properties of Fusarium infected bananas. J Phytopath 165:213–222
Bhardwaj A, Sharma D, Jodan N, Agrawal PK (2015) Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Arch Clin Microbiol 6:1–9
Bolouri Moghaddam MR, Vilcinskas A, Rahnamaeia M (2016) Cooperative interaction of antimicrobial peptides with the interrelated immune pathways in plants. Mol Plant Pathol 17:464–471
Campos ML, Kang JH, Howe GA (2014) Jasmonate-triggered plant immunity. J Chem Ecol 40:657–675
Chandrasekaran M, Chun SC (2016) Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. carotovora. Biosci Biotechnol Biochem 80:2277–2283
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants-with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Chuankun X, Minghe M, Leming Z, Keqin Z (2004) Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol Biochem 369:997–2004
Chun SC, Paramasivan M, Chandrasekaran M (2018) Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front Microbiol 9:2525
Chung S, Kong H, Buyer JS, Lakshman DK (2008) Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soil borne pathogens of cucumber and pepper. Appl Microbiol Biotechnol 80:115–123
Dennis C, Webster J (1971) Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Trans Br Mycol Soc 57:41–44
Dunlap CA, Bowman MJ, Rooney AP (2019) Iturinic lipopeptide diversity in the Bacillus subtilis species group-important antifungals for plant disease biocontrol applications. Front Microbiol 10:1794
Elanchezhiyan K, Keerthana U, Nagendran K, Prabhukarthikeyan SR (2018) Multifaceted benefits of Bacillus amyloliquefaciens strain FBZ24 in the management of wilt disease in tomato caused by Fusarium oxysporum f. sp. lycopersici. Physiol Mol Plant Pathol 103:92–101
Fabro G, Kovacs I, Pavet V, Szabados L (2004) Proline accumulation and AtP5CS2 gene activation are induced plant pathogen incompatible interactions in Arabidospis. Mol Plant Microbe Interact 17:343–350
Gay PA, Tuzun S (2000) Temporal and spatial assessment of defense responses in resistant and susceptible cabbage varieties during infection with Xanthomonas campestris pv. campestris. Physiol Mol Plant Path 57:201–210
Jain A, Singh S, Kumar Sarma B, Bahadur Singh H (2012) Microbial consortium–mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J Appl Microbiol 112:537–550
Kets EPW, Galinski EA, de Bont JAM (1994) Carnitine: a novel compatible solute in Lactobacillus plantarum. Arch Microbiol 162:243–248
Khan N, Martínez-Hidalgo P, Ice TA, Maymon M, Humm EA, Nejat N, Sanders ER, Kaplan D, Hirsch AM (2018) Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol 9:2363
Lavania M, Chauhan PS, Chauhan S, Singh HB (2006) Induction of plant defense enzymes and phenolics by treatment with plant growth-promoting rhizobacteria Serratia marcescens NBRI1213. Curr Microbiol 52:363
Leon IP, Montesano M (2013) Activation of defense mechanisms against pathogen in mosses and flowering plants. Int J Mol Sci 14:3178–3200
Li L, Steffens JC (2002) Over expression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247
Liang X, Zhang L, Natarajan SK, Becker DF (2013) Proline mechanisms of stress survival. Antioxid Redox Signal 19:998–1011
Liu TT, Wu P, Wang LH, Zhou Q (2011) Response of soybean seed germination to cadmium and acid rain. Biol Trace Elem Res 144:1186–1196
Mejía LC, Herre EA, Sparks JP, Winter K (2014) Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol 12:479
Mukherjee PK, Horwitz BA, Singh US, Mukherjee M (2013) Trichoderma: biology and applications. CABI 2013:16
Oney-Birol S (2019) exogenous L-carnitine promotes plant Growth and cell Division by Mitigating Genotoxic Damage of Salt Stress. Sci Rep 9:1–12
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM (2014) Induced systemic resistance by beneficial microbes. Ann Rev Phytopathol 52:347–375
Ramarathnam R, Bo S, Chen Y, Fernando WD (2007) Molecular and biochemical detection of fengycin-and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol 53:901–911
Samsatly J, Copley TR, Jabaji SH (2018) Antioxidant genes of plants and fungal pathogens are distinctly regulated during disease development in different Rhizoctonia solani pathosystems. PLoS ONE 13:e0192682
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31:446–459
Shimizu T, Watanabe M, Fernie AR, Tohge T (2018) Targeted LC-MS analysis for plant secondary metabolites. In Plant Metabolomics Humana Press, New York, NY 171–181
Shoresh M, Harman GE (2008) The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: a proteomic approach. Plant Physiol 147:2147–2163
Solanki MK, Robert AS, Singh RK, Kumar S, Pandey AK, Srivastava AK, Arora DK (2012) Characterization of mycolytic enzymes of Bacillus strains and their bio-protection role against Rhizoctonia solani in tomato. Curr Microbiol 65:330–336
Tagg J, McGiven AR (1971) Assay system for bacteriocins. Appl Microbiol 21:943
van der Ent S, Van Wees SC, Pieterse CM (2009) Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochem 70:1581–1588
Van Loon LC (2007) Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 19:243–254
Wang P, Zhang S, Wang C, Lu J (2012) Effects of Pb on the oxidative stress and antioxidant response in a Pb bioaccumulator plant Vallisneria natans. Ecotoxicol Environ Saf 78:28–34
Wen PF, Chen JY, Kong WF, Pan QH (2005) Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci 169:928–934
Xie JH, Chai TT, Xu R, Liu D (2017) Induction of defense-related enzymes in patchouli inoculated with virulent Ralstonia solanacearum. Electron J Biotechnol 27:63–69
Yasmin S, Zaka A, Imran A, Zahid MA, Yousaf S, Rasul G et al (2016) Plant growth promotion and suppression of bacterial leaf blight in rice by inoculated bacteria. PLoS ONE 11:e0160688
Yuan J, Raza W, Shen Q, Huang Q (2012) Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. Cubense Appl Environ Microbiol 78:5942–5944
Zalila-Kolsi I, Mahmoud AB, Ali H, Sellami S (2016) Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol Res 192:148–158
Acknowledgements
The authors thank UTU management for providing B. U. Patel research fellowship to carry out this work. Authors also acknowledge IIT Bombay SAIF for providing LC–MS analysis service.
Author information
Authors and Affiliations
Contributions
NA: Designed the work. HNJ: Performed the experiments. KS: Performed AMP gene amplification work. HNJ and NA: Data analysis and drafting the article.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
All the authors read the manuscript and gave their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jinal, H.N., Sakthivel, K. & Amaresan, N. Characterisation of antagonistic Bacillus paralicheniformis (strain EAL) by LC–MS, antimicrobial peptide genes, and ISR determinants. Antonie van Leeuwenhoek 113, 1167–1177 (2020). https://doi.org/10.1007/s10482-020-01423-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-020-01423-4