Abstract
Trimeric autotransporter adhesins (TAAs) are located on the surface of many pathogenic Gram-negative bacteria. TAAs belong to the autotransporter protein family and consist of three identical monomers. These obligate homotrimeric proteins are secreted through the bacterial type Vc secretion system and share a common molecular organization that each monomer consists of a N-terminal “passenger” domain and a C-terminal translocation domain. TAAs are important virulence factors that are involved in bacterial life cycle and participate in mediating infection, invasion, dissemination and evasion of host immune responses. TAAs have also proved to be useful for many applications, such as vaccines and disease biomarkers. We here mainly focused on new findings on bio-function and application of TAAs in addition to their common structure and secretion mechanisms.
Similar content being viewed by others
Introduction
Trimeric autotransporter adhesins (TAAs) belong to a growing group of surface adhesins secreted by several types of pathogenic Gram-negative bacteria and involved in pathogenesis, as well as in the defense against host immune responses. The earliest described and most prototypical TAA is YadA from Yersinia enterocolitica and Yersinia pseudotuberculosis (Bolin et al. 1982; Skurnik et al. 1984); other prominent examples of TAAs include Hia and Hsf from Haemophilus and NadA from Neisseria meningitidis. As TAAs are homologous multimers containing three polypeptide chains, each monomer makes the same contribution to the complete structure. All TAA family members share a common molecular architecture: a multifunctional, long “passenger” domain at the N-terminus and followed by a stable, highly conserved C-terminal translocation domain or anchor domain (Linke et al. 2006). Each of these two domains has its unique architectures and bioactivities. Additionally, the immature TAA proteins have a signal peptide, most of which contain approximately 20–30 amino acids, only 10 % of them contain more than 50 amino acids (Dautin and Bernstein 2007). TAAs have attracted significant attention since their discovery on the Y. enterocolitica surface. In addition to YadA, Hia and Hif, recent studies have suggested that several additional life-threatening infectious Gram-negative bacteria possess one or more TAAs; these proteins include Apa from Actinobacillus pleuropneumoniae (Xiao et al. 2012), SadA from Salmonella enterica (Raghunathan et al. 2011), BpaA from Burkholderia pseudomallei (Edwards et al. 2010), BadA from Bartonella (Riess et al. 2004; Szczesny et al. 2008), EmaA from Actinobacillus actinomycetemcomitans (Mintz 2004), and Cha from Haemophilus (Sheets et al. 2008).
In recent years, the biological role of TAAs and their corresponding mechanisms have been fully studied. The canonical function of TAAs is their adhesion activity. To adapt to a changing environment, TAAs not only can adhere to non-living materials (Ishikawa et al. 2014), such as plastic and glasses, but also can adhere to living objects, including mediating bacterial attachment to host cells and mediating interactions between bacteria that results in bacterial autoaggregation or biofilm formation. There is accumulating evidence suggest that TAAs play key roles in bacterial pathogenesis. TAAs can promote bacterial infection, colonization, invasion and dissemination via bacterial attachment to the extracellular matrix (ECM), cell surface receptors or host tissues. TAAs are also involved in the evasion of host immune responses via anti-serum or by resisting phagocytosis. More importantly, recent studies have indicated that TAAs not only can function alone, but also interact with other bacterial virulence factors.
Although TAA family members have similar spatial structures, many details remain unknown due to the complexity of TAA structure and function and variability between bacteria species. However, it is exciting that there is a bright future in the possible applications of TAAs in disease control in addition to their important virulence functions. Many studies have reported that TAAs have potential as vaccine candidates and in clinical diagnosis. Most existing TAAs reviews focus on TAAs structure and secretion mechanisms; therefore, this review places more emphasis on the current knowledge on new findings and potential applications of TAAs in addition to simply reviewing their common structures and secretion mechanisms. In addition, we suggest the future work need to solve the impending questions and to fully understand the roles of TAAs in bacterial disease.
The common molecular structures of TAAs
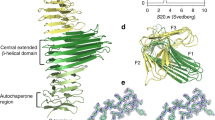
All TAA family members are composed of three identical polypeptide chains and share a common architecture feature, despite a limited degree of sequence similarity. As shown in Fig. 1, the head-stalk-anchor architecture of each monomer consists of a C-terminal translocation membrane-anchored domain and an N-terminal head-surface-exposed passenger domain. In addition, there is a signal peptide located at the N-terminus of the passenger domain in pre-autotransporters. The passenger domain consists of the following three sub-domains: the adhesive head domain, the neck connector domain and the long, repetitive stalk domain. Each area in this passenger domain has a unique structure and bioactivities. The head domain, for example, mediates the adhesion function of TAA family members in most cases. The stalk projects the functional head away from the bacterial cell surface. The short neck domain acts as an adaptor and connector between the head domain and stalk domain. The C-terminal translocation domain contains approximately 70 amino acids (Roggenkamp et al. 2003; Surana et al. 2004) that embeds into the outer membrane to anchor the TAA protein in the bacterial membrane and acts as a channel for the passenger domain translocation. This translocation domain is so highly conserved throughout TAA family members that defines this family. Among this growing family, YadA and Hia are the two most well-characterized TAAs and have become models for research on other TAA family members (Fig. 2).
The common structure of TAAs. The blue area represents the head domain. The red domains represent repetitive sequences in the stalk domain. The green domain represents the anchor at the COOH-terminus. The turquoise area at the NH2-terminus represents signal peptides and is followed by a cleavage site, which are represented by the pink area. Between the head and stalk domain is the neck domain; and the neck-like domain is located between the COOH-terminal anchor and the stalk domain
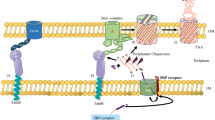
The TAA transmembrane translocation process. The pink, grey and purple regions represent TAA monomers. A describes the signal peptides mediated Sec-complex-dependent translocation across the inner membrane (IM). B, C, and D describe the process of crossing the outer membrane (OM). B represents the initial step. The C-terminus is embedded in the OM and forms a β-stand pore, which acts as a channel for the transport of passenger domains. C represents the middle step. The passenger domains translocate from the β-stand pore to the surface of the bacteria cell. The arrows represent the translocation direction of passenger domains. D represents the final step. After the translocation process is complete, the protein is anchored to the cell surface. The adhesive head is projected away from the cell surface by the long stalk domain, and the beta-stand pore is blocked by the C-terminal passenger domain. The Bam complex (blue) assists the B, C, D process in some degree
The structure state of TAA proteins may display differently on the cell surface during different phases of the bacterial life cycle or between different family members. Most TAAs are nonlinear on the surface due to their specific structures, such as the “hairpin-like” twisted molecules model as an example, which results in shorter proteins than predicted based on their amino acid sequence (Singh et al. 2014a).Though some previous studies have suggested some TAAs appear as straight, fibre-like structures on the cell surface, they become bent once they bind to host ligands (Agnew et al. 2011; Ishikawa et al. 2014).
The common structure of head domains
In most cases, the head of TAA proteins share a common structure with that of YadA, therefore those domains were named as YadA-like heads. The crystal structure of this domain was recently solved by X-ray crystallography and showed this domain consists of a left-handed parallel beta-roll lollipop-like structure with an internal hydrophobic core followed by the coils of the adjacent neck domain, which form a very stable “lock nut” that ties the oligomer together in a clockwise manner (Nummelin et al. 2004). Within these common features, there exist exceptions. Szczesny et al. (2008) found that the head of Bartonella BadA consists of two additional domains, the “Trpring domain” and the “GIN domain”. These domains are not similar to any known structures but appear to be a chimaera of domains seen in YadA and Hia. The Trp-ring-GIN tandem may harbour fibronectin binding sites (Szczesny et al. 2008). Recently, a more complex TAA was found on the surface of Burkholderia pseudomallei, BpaA, whose head structure exhibits a novel fold of an intricately interwoven trimer that contains modular structural elements from other TAAs (Edwards et al. 2010), highlighting the combinatorial evolutionary strategy taken by these pathogens (Fig. 3).
TAA-mediated bacterial infection and invasion. TAAs mediate bacterial attachment to the host cell by adhesion to the ECM (via collagen, laminin, fibronectin, and heparin sulphate) and extracellular HSP90 proteins then activate intercellular signal pathways to produce cell responses. TAAs mediate cell invasion by directly binding to cell surface receptors (CEACAM1 or β1-integrin receptor) or by contact with clathrin to induce cytoskeleton re-arrangement or induce host cell informatory response by activation of MAPK and NF-κB signaling pathway
The common structure of stalk domains
Following the head domain is the long, sequence repetitive stalk domain, which is the largest domain in TAA proteins. X-ray crystallographic studies have suggested that almost all TAA stalks contain multiple right-handed coiled-coil repeats, which are followed by periodicities left-handed segments with sequence periodicity. Between these two segments are several copies of the sequence motif YxD. This motif is characteristic of the right-handed coiled coils of TAAs (Szczesny and Lupas 2008); two of this motif in the conserved transition region between the right-handed coiled-coil segment and the left-handed segments play a role in specifying the oligomerization state and increase the stability of trimeric right-handed coiled coils (Alvarez et al. 2010). The repetitive structural units in the stalk domain elongate the entire autotransporter structure on the cell surface.
The common structure of translocation domains
Following the long stalk domain is the translocation domain, which embeds into the bacterial outer membrane. This C-terminal translocation domain generally consists of about 70 amino acids and functions as an anchor, holding the translocated passenger domain on the bacterial surface and earning the name “anchor domain”. The amino acid sequence of this domain is highly conserved within the TAA family and is used as the defining element of the TAA family (Cotter et al. 2005). Likewise, the crystal structure of the translocation domain is also highly similar that forms a β-barrel pore containing 12 transmembrane β-strands that each monomer contributes four strands (Meng et al. 2006). These pores act as channels for transporting the three N-terminal α-helices passenger domains through the bacterial outer membrane (Roggenkamp et al. 2003; Oomen et al. 2004; Surana et al. 2004; Meng et al. 2006). Shahid et al. predicted the secondary sturcture of YadA-M by taking advantage of solid-state magic-angle spinning (MAS) NMR and indicated that this domain contains a number of amphipathic, antiparallel β-strands, at both ends of these β-strands are frequently made from aromatic residues that interact with the water–lipid interface to anchor the TAAs in the lipid membrane (Shahid et al. 2012).
Domains connecting sub-domains
Though the common architecture of TAAs is a head-stalk-anchor model, each functional group cannot exist independently. Instead, functional groups are linked by a number of smaller structures to form a continuous biomacromolecule. Firstly, between the head and stalk domains is a neck domain with conserved residues. According to their length, these necks are subdivided into short necks, long necks, and ISneck (necks with an insertion sequence) (Hartmann et al. 2012). The neck functions as a connector and adapter to smoothly transition from the large α-helical coiled globular head domain to the narrow β-coiled-coil stalk domain (Lyskowski et al. 2011). This neck domain also acts as a “safety pin” holding the three monomers together that in part explains the stability of trimeric proteins; the involvement of the neck domain in stalk formation and protein folding on the cell surface helps maintain the architecture of TAAs (Nummelin et al. 2003). In addition, there also exist some domains tandem with neck domains, such as the DALL domains in SadA (Hartmann et al. 2012). Likewise, the existence of an intertwined domain similar to the neck domain between the head and passenger domains was recently reported; this domain is ubiquitous among trimeric autotransporters and acts as the structural transition between the passenger domain and the translocation domain (Meng et al. 2008). Noteworthy, within in each domain may also exist min-connectors at the interface of different helixes,take the joins or inserts within the stalk of EibD as an example (Leo et al. 2011).
The influencing factors on the structure of TAAs
TAAs are large multimeric bioactive proteins on the cell surface raising the following question: how can a trimer be so stabile without stabilizing disulphide bridges between monomers? Previous studies have indicated that both TAA proteins themselves and other modifications have positive impacts on the stability of TAAs and their spatial structure. Firstly, the twist between the righted-hand coiled coils and the left-hand coiled coils in the stalk may be advantageous for folding (Leo et al. 2011); the polar core residues in the coiled-coil domain sequester anions to the hydrophobic core and form a highly ordered network of polar interactions, which help maintain the coiled coils in a soluble and unfolded state during the export process (Hartmann et al. 2009). The C-terminal translocation domain plays so a determining role in maintaining trimerization and ability that even a single amino acid mutations can affect trimerization, surface localization, and consequently function of TAA proteins (Echenique-Rivera et al. 2011).Thirdly, the hydrophobic core of the head together with the coils of the neck hold the oligomer together, and, meanwhile, the multi-ion networks between subunits largely contribute to stabilize the protein (Nummelin et al. 2004). Finally, the modifications of TAA proteins, such as glycan modifications, may contribute to architecture stability and collagen binding activity (Tang et al. 2012).
It can be concluded that the TAA family members share a highly similar secondary structure, described as a head-stalk-anchor domain architecture. The passenger domain is anchored to the surface after being translated; its head domain is a left-handed β-roll lollipop-like structure and is projected away from the cell surface by the long repetitive coiled-coils stalk domain. The translocation domain is highly conversed among TAA proteins and is used to define TAA family members. This domain forms a β-barrel pore containing 12 trans-membrane β-strands within the bacterial outer membrane. The connector domains located between the sub-domains make these long proteins smooth and continuous. As functional proteins, stable structural conformations of the sub-domains contribute to varying degrees to the bioactivity of TAAs.
The translocation process of TAAs
Functional outer membrane proteins must be transported to the membrane surface after being synthesized in the cytoplasm. TAAs, without exception, are transported to the cell surface through bacterial type Vc secretion systems to display their bio-functions. In this simple secretion system, these secreted proteins transport across the bacterial membranes in a two-consecutive step process (Henderson et al. 2004). Firstly, the N-terminal signal peptide mediates the subunit crosses the inner membranes in a Sec-dependent manner (Linke et al. 2006; Dautin et al. 2007), leading the passenger domain to the periplasm in an unfolded state (Dautin and Bernstein 2007). Once this step is complete, the signal peptide is cleaved off from the N-terminus and the passenger domains then fold into transport-incompetent conformations in the periplasm. Meanwhile, the pre-TAAs are modified within the periplasm by processes such as glycosylation (Tang et al. 2012). Secondly, the passenger domain is then translocated through the outer membrane in a C- to N-terminal direction (Bernstein 2007; Junker et al. 2009; Peterson et al. 2010), and projects from the cell surface. But the elaborate mechanisms that drive this sophisticated process remain poorly understood.
Study of the secretory mechanism of TAAs will not only help us fully understanding the TAA protein family, but also such research is important for the prevention and treatment of bacterial diseases. However, this kind of work is full of challenges so that there still exist many controversies in TAAs secretion. It is undeniable that the anchor domain is a prerequisite that plays an important role in translocating through the outer membrane. As reviewed above, the anchor domain embeds into the outer membrane surface to form a β-barrel pore, a relatively rigid structure held together by an extensive network of hydrogen bonds formed between the individual β-strands (Dautin and Bernstein 2007) and stabilized by the N-terminal loop region (Meng et al. 2006). It is this specific pore-like structure that allows the anchor domain to function as an autotransporter, transporting its own N-terminal domain across the membrane. Single point mutations in this transport pore can slow or fully inhibit the translocation process (Grosskinsky et al. 2007). However, accumulating evidence indicates that at least some passenger domains may fold in the periplasm and remain folded during the translocation process (Veiga et al. 1999) which would require additional space in the β-barrel pore during secretion. In addition, the recently determined crystal structure of the Hia protein raises a question as to how the “large” primary binding domain (Yeo et al. 2004) passes through the 1–2-nm pore size of the classic autotransporter β-domains. All these newly findings challenge this automatic process and indicate that other existing molecules may assist the translocation process. The following studies did find that this secretion process requires assistance from other molecules. A number of models suggest that anchor domain insertion into the outer membrane requires the integration of Bam machinery for outer membrane localization and the surface presentation of the passenger domain (Lehr et al. 2010). Additional studies have suggested the Bam complex interacts with the β-barrel and passenger domains of autotransporters at multiple locations and holds the trimeric β-barrel pore in an open configuration, as proposed in the monomeric secretion model, creating enough space for the secretion of all three subunits until the passenger domain is fully translocated (Bernstein 2007; Peterson et al. 2010). This may explain how the large trimeric passenger domain passes through the small β-barrel pore. Furthermore, Lehr et al. found that BamA interacts directly with the C-terminal amino acid motif of YadA and is essential for the biogenesis of the YadA. They also suggested that the Bam complex plays an important role in TAA protein translocation and folding (Lehr et al. 2010). Obviously, the relationship between the anchor domain and the Bam complex seem to be that the C-terminal β-barrel domain serves as a targeting signal for the Omp protein complex (Oomen et al. 2004). In turn, the Omp complex facilitates the formation and insertion of β-barrels into the outer membrane (Voulhoux et al. 2003). The signal peptide is also reported to contribute to the TAAs translocation process in addition to leading the proteins across the inner membrane (Jiang et al. 2011). Removement or replacement of the signal peptide of EspP, an autotransporter of Escherichia coli that owns a long signal peptide, with a generic signal peptide impaired the translocation of the passenger domain across the OM, highlighting the importance of these specific signal peptides in maintaining the passenger domain in a suitable state for translocation across the OM by preventing its misfolding (Szabady et al. 2005). Noteworthy, many periplasmic chaperone or protease also involved in the translocation of the TAAs; bacterial periplasmic chaperone Skp enhances the proper assembly of trimeric autotransporter (Ulrich et al. 2014), the chaperone protease DegP degrades the accumulated pre-TAAs in the periplasm caused by mutation, which is important for correct biogenesis of TAAs (Grosskinsky et al. 2007). Interestingly, Iwan Grin et al. recently found a small trimeric inner membrane lipoprotein with direct influence on the structural integrity of SadA in the periplasm, suggesting that there may exist additional periplasmic proteins that assist autotransport process (Grin et al. 2014).
We can conclude that the “autotransport” process does not tend to be independent. Though the C-terminal anchor domain is crucial for multimerization and is a prerequisite for insertion into the outer membrane and presenting adhesin on the cell surface, the assistance of the Bam complex or other molecules are needed. Their relationship seems to be that the anchor domain forms β-barrels that are required for pore formation, which acts as a channel for translocation. While, the Bam complex is involved in facilitating the initial step of translocation by promoting the insertion of β-domain into the lipid bilayer and stabilizing the open conformation of the β-barrel during the whole translocation to allow for the passage of the partially folded passenger domain (Oomen et al. 2004; Meng et al. 2006). Once export is complete, the chains fold and the stalk forms a 3-stranded coiled coil that extends into the pore to occlude its opening (Hartmann et al. 2009). Notably, there may still exist other potential unknown molecules that also assist with TAA translocation. It should be pointed out that this secretory mechanism is still havenot been fully revealed due to the complexity of the TAAs secretion process and the limit of research methods. Although we have recognized this autotranslocation process needs the assistance of other protein molecules, we still do not know how much this process depends on these extra proteins and whether TAAs can be partially or completely translocated in the absence of these molecules.
New findings on the function and related mechanisms of TAAs
In addition to mediating adhesion to the extracellular matrix and to host cells, TAAs are also involved in various pathogenic processes of Gram-negative bacteria, including biofilm formation, autoaggregation, and other important pathogenesis-related processes, such as cytotoxicity, serum resistance, host cell invasion, and survival within host cells (Mil-Homens and Fialho 2011).
New findings on TAA-mediated bacterial attachment: the canonical function
Adhesion is the canonical function of TAA proteins. Previous studies have demonstrated that TAAs can adhere not only to abiotic materials (Ishikawa et al. 2014), such as glass and plastics, but also can adhere to the ECM, host cells and tissues, and that the possible binding sites of TAAs. Several studies have indicated that the head domain is the primary mediator of attachment. However, many other sites are also related to bacterial adherence. The stalk domain plays a crucial role in fibronectin (Fn) binding (Kaiser et al. 2008, 2012); the C-terminal passenger domain, together with the translocation domain could adhere to the ECM, fibronectin and vitronectin (Leduc et al. 2009). These studies demonstrated that the head, stalk and translocation domains share overlapping functions. With description of binding sites available, many new ligands were also identified. Many groups found that TAAs can directly interact with receptors on different cell surface to promote invasion. Hia mediates high-affinity adherence to respiratory epithelial cells (Laarmann et al. 2002). EhaG and UpaG mediate specific adhesion to colorectal epithelial cells and bladder epithelial cells, respectively (Totsika et al. 2012). Burkholderia cenocepacia TAA proteins can recognize receptors on the respiratory epithelium and living pneumocytes to form membrane tethers that behave as stiff nanosprings to promote bacterial adhesion to cells, favoring host colonization (El-Kirat-Chatel et al. 2013). UspA1 binds to the human CEACAM1 receptor, an immunoglobulin (Ig)-related glycoprotein, or binds host cells through its stalk binding sites, promoting the invasion of Moraxella (Hill et al. 2005; Conners et al. 2008). Furthermore, many new receptors on the cell surface of host tissues were recently detected, for example, NadA can interact with β1-integrin and the heat shock protein Hsp90 (Cecchini et al. 2011; Montanari et al. 2012; Bozza et al. 2014) to adhere to and invade host cells. TAAs can also interact with themselves, resulting in the formation of microcolonies and autoaggregation, which can eventually lead to biofilm formation, another mechanism of immune evasion (Spahich and St Geme 2011).
It has been established that TAAs attach to themselves and bind to the ECM. In addition, although their exact structural conformations and their underlying molecular mechanisms remain poorly understood, the potential binding sites of TAAs have gradually been recognized. The available data indicates that adhesion is largely associated with amino acid sequences in binding regions, as studies have shown that single amino acid changes in binding sites in the head (Radin et al. 2009) or the highly conserved neck domain (Roggenkamp et al. 2003) alter binding affinity. Binding affinity is also closely related to the trimeric state and spatial conformation of TAA proteins. Researchers have found that the receptor-binding site in the head domain has a trimeric architecture with threefold symmetry and a mushroom shape formed by an acidic patch from all three monomers. These acidic regions allow for potential multivalent interactions with the host cell surface, increasing avidity and stabilizing bacterial adherence to overcome host physical forces (Yeo et al. 2004). Tahir et al. described the structural details of the binding site and pointed that the NSVAIG-S motif in the hydrophobic core and the conformational three-dimensional structure of TAA is required for ligand binding (Tahir et al. 2000). A complete trimeric conformation is necessary for full adhesion ability in TAAs. Previous studies examined the relationship between trimerization of the passenger domains and the capacity for adhesion activity demonstrating that subunit–subunit interactions and stable trimerization are essential for native folding, stability and full-level adhesion activity in Hia and YadA (Cotter et al. 2006). In contrast, the perturbation of subunit–subunit interactions results in the disruption of the trimer and loss of adhesion ability (Radin et al. 2009). Schutz et al. also found that trimer stability in the YadA protein is essential for virulence (Schutz et al. 2010). Surprisingly, the length of the repeated stalk domain also interferes with adhesion to host cells and bacteria cells themselves. Changes in this region effect the adaptive capabilities of bacteria (Sheets and St Geme 2011).
Previous research has suggested that TAAs share some common adhesion traits. Firstly, almost all TAAs can tightly adhere to matrix components and host cells under both static and dynamic flow conditions (Muller et al. 2011), and moreover this attachment can withstand high forces (El-Kirat-Chatel et al. 2013). Secondly, many TAAs contain more than one binding site. Daigneault et al. found that AhsA from Mannheimia haemolytica A1 contains more than 21 collagen-binding motifs as identified by sequence analysis (Daigneault and Lo 2009); the Hia and Hif proteins contain 2 and 3 binding sites, respectively (Meng et al. 2008; Singh et al. 2014a). Finally, for interaction between collagen and TAAs, binding capacity can be affected by both collagen and state of TAAs. The specific structure of collagen is necessary for TAA binding to in a triple-helical conformation-dependent manner; however, sequence specificity of collagen appears to be minimal (Leo et al. 2008). Meanwhile, the conformation state of TAAs, as mentioned above, greatly influences adhesion capacity.
New findings related to TAA-mediated bacterial resistance to host serum
Another important role of TAA is in resistance to host serum which mainly mediated by the stalk domain (Ackermann et al. 2008; Leduc et al. 2009). Further research has determined that the C-terminal translocator domain also contributes to serum resistance (Ackermann et al. 2008), moreover, only minor changes in the membrane anchor in YadA directly or indirectly affect complement resistance (Schutz et al. 2010). Binding to immunoglobulin G (IgG) and immunoglobulin M (IgM) via TAA not only contributes to the inhibition of opsonization but also may prevent the activation of the classical complement pathway by blocking the Fc binding site for C1q; this allows bacteria thus to evade the host immune response (Abdullah et al. 2005; Leo et al. 2011). In addition, binding to the complement factor H, complement C3 or complement binding protein C4 bp in the stalk domain can result in serum resistance by inhibiting the complement alternative pathway. By inhibiting C5b–C7 complex formation and C9 polymerization, the formation of membrane attack complexes (MACs) is inhibited (Attia et al. 2006; Hallstrom et al. 2006; Singh et al. 2014b). Noteworthy, several conformational and discontinuous sites, rather than a restricted sequence in the stalk domain contribute to this binding effect (Aebi et al. 1998; Nordstrom et al. 2004; Biedzka-Sarek et al. 2008). Surprisingly, TAAs can also mediate bacterial resistance to host serum indirectly by binding to ECMs or via the physical effects of TAAs in addition to directly by binding to complements. For examples, binding of Vitronectin to Moraxella catarrhalis UspA2 or Haemophilus influenza Hib interferes with the proper formation of the membrane attack complex (MAC) in bacterial outer membranes, that lead to bacteria obtain serum resistance (Attia et al. 2006; Hallstrom et al. 2006); binding fibronectin and vitronectin to DsrA contributes to the high-level serum resistance in Haemophilus ducreyi (Leduc et al. 2009); TAAs at the surface may physically shield epitopes necessary for complementation on the surface of the bacteria, further contributing to serum resistance (Biedzka-Sarek et al. 2008; Leduc et al. 2009).
New findings on the multiple functions of TAAs
Apart from adhesion activity and anti-serum effects, TAAs are also involved in many other important pathogenic processes of bacteria. Recent study suggested that TAAs are involved in remodeling the host cellular membrane and promoting the bacterial invasion of non-phagocytic cells via the polymerization of actin and influence on cytoskeleton remodeling and clathrin (Serruto et al. 2009), although the receptor on the cell surface that would mediate such an effect is still unknown. Like many other important virulence factors, TAAs can induce host inflammatory response. Studies have demonstrated that YadA can induce the secretion of interleukin-8 (IL-8), a potent chemoattractant of polymorphonuclear leukocytes, by activation of MAP kinases and activation of the transcription factor NF-κB, which then promotes host inflammatory reactions (Schmid et al. 2004; Eitel et al. 2005; Schutz et al. 2010). Similarly, we recently found that Adh, the head domain of Apa, enhanced pathogenicity of A. pleuropneumoniae to piglets by increasing the release of pro-inflammatory cytokines and depressing host immune recognition system(Wang et al. 2015b). More importantly, Adh may be also involved in A. pleuropneumoniae mediated the apoptosis of porcine primary alveolar macrophages (Wang et al. 2015a). TAA are involved in the evasion of host immune responses and dissemination throughout the host. In addition to in resistance towards serum as reviewed above, TAAs can protect bacteria from phagocytosis by macrophages (Sjolinder et al. 2008) and circumvent neutrophilic attack (Paczosa et al. 2014), allowing for evasion from the host’s innate immune response and directly promoting persistent bacterial infection. Additionally, biofilm formation resulted from interactions between TAAs, also mediate immune evasion. Noteworthy, TAAs also exert effects on other bacterial virulence factors. Yun et al. found that the Bartonella henselae BadA and the VirB/D4 type IV secretion system can influence each other at different phases (Lu et al. 2013).
Collectively, TAAs act as important virulence factors. On one hand, TAAs can increase the internalization of bacteria into host cells and the colonization of host tissues by binding to ECM or the cell surface in both specific and non-specific adhesion; on the other hand, TAAs can also promote bacteria to evade the host immune response by resisting serum, avoiding phagocytosis and other bioactivities. Many new functions and cell receptors have been recently identified, contributing to our understanding of virulence of TAAs. What’s more, TAAs can impact bacterial virulence on their own or interact with other virulence factors.
Potential applications of TAAs
Having a clear understanding of the role of TAAs in virulence, many groups have explored the potential applications of these proteins and their results are encouraging. TAAs can be used as biomarkers of some diseases. More importantly, beside TAAs themselves are potential vaccine components, their secretory system can also be used in vaccine design.
TAAs can be used as potential molecular biomarkers for diagnosis. Mil-Homens et al. found that three TAA-encoding genes out of more than 74 TAA-encoding sequences in B. cepacia complex (Bcc) genomes were expressed during the infection process; the products of these genes are candidates for multifunctional virulence factors (Mil-Homens and Fialho 2011). Tiyawisutsri et al. further identified four additional TAAs in B. mallei that were specially expressed during glanders infection but not in melioidosis (Tiyawisutsri et al. 2007). We previously reported that the Apa gene from A. pleuropneumoniae was upregulated during infection to piglets primary pulmonary alveolar macrophage (PAMs) in vitro (Wang et al. 2014a). Taken together, these statistics indicate that TAAs are upregulated during infection process both in vivo and in vitro and further can be used as potential molecular biomarkers for diagnosis.
TAAs possess good immunogenicity and effective immune protection, indicating these proteins are potential vaccine candidates. As attachment of bacteria to host cells is a preliminary step in pathogenesis and TAAs play an irreplaceable role in this preliminary step, thus effectively inhibition or neutralization of their function can inhibit adhesion to the host which would further attenuate the odds of infection. Therefore, the use of TAAs as vaccine ingredients seems to be an effective strategy to reduce or prevent bacterial infections. Surprisingly, studies completed both in mice models and natural hosts proved TAAs are immunogenic and can effectively protect against bacterial infections. The Hap autotransporter from H. influenzae was found to be immunogenic in mice and can protect against diseases caused by non-typeable H. influenza (Cutter et al. 2002). Two groups have shown experimental evidence that NhhA can induce bactericidal antibodies that can protect mice against meningococci (Weynants et al. 2007; Tavano et al. 2011). UspA1 and NadA induce antibody production in natural hosts and prevents the binding of M. catarrhalis to the cell receptor CEACAM1, decreasing the odds of infection (Hill et al. 2005). The presence of anti-AhsA antibodies can be detected in calves challenged with M. haemolytica A1, suggesting that AhsA is immunogenic in cattle (Daigneault and Lo 2009). Fusco et al. demonstrated that immunization with the passenger domain of DsrA protects swine against Haemophilus ducreyi infection (Fusco et al. 2014). SadA has been shown to have limited protective function (Raghunathan et al. 2011). It has been suggested that ExPEC and UpaG can be used as protective antigens against pathogenic E. coli (Durant et al. 2007). The TAA protein HA from Avibacterium paragallinarum can protect against infectious coryza (Wang et al. 2014b). NadA is capable of promoting dendritic cell maturation and may be used as a self-adjuvant (Mazzon et al. 2007). Taken together, these studies suggest that TAAs are immunogenic antigens both in model animals and natural hosts. Moreover, further studies suggest that TAAs contain multiple antigenic epitopes that appear primarily at the N-terminus (Tahir et al. 2000). Therefore, TAAs are excellent candidate molecules for vaccines. Even though the immunological functions of TAAs are still being researched, many TAAs have been successfully used as vaccine components with positive effects. NadA, whose protective conformational epitopes are located in the head domain, is upregulated and immunogenic during infection (Fagnocchi et al. 2013); the use of this protein as a component of a multicomponent vaccine was approved by the European Medicines Agency in early 2013 (Malito et al. 2014). Previously, the Bordetella pertussis autotransporter adhesin, a Type Va autotransporter known as Pertactin, has been successfully used as a component of the multicomponent vaccine (Wells et al. 2007). All these results indicated the autotransporter adhesins, including monomeric autotransporter adhesins and trimeric autotransporter adhesins are promising vaccine candidates.
In addition to their potential use as vaccine ingredients, the secretion system of TAAs can also be used in vaccine development. Firstly, previous studies indicated the bacterial Type Va secretion system can be used as efficient autodisplay platform for the development of multivalent recombinant bacterial vector vaccines (Jong et al. 2014; Kranen et al. 2014); The TAAs secretion system, Type Vc secretion system, also shares this autodisplay characteristic, indicating it can be used as a display platform for the efficient recombinant subunit vaccine development. More importantly, autodisplayed vaccine can effectively increase the immunogenicity of recombinant antigens (Jose 2006). Therefore, the type Vc secretion system may play an important role in the development of novel subunit vaccine in the future. Secondly, as the immunogenicity of multimeric forms of proteins can be stronger than that of monomeric proteins (Pantophlet and Burton 2003) and this secretion system can promote the trimerization of proteins, it is an ideal strategy to use this secretion system to polymerize monomers into trimers to boost the immunogenicity of many candidate vaccine proteins.
Remarks on future research directions
Since the first discovery of TAA proteins, more and more TAAs have been identified on the surface of bacterial pathogens. In past years, more details concerning these proteins have been revealed, especially on the structure, function and secretion mechanisms and many new functions and new mechanisms in the infection process were identified. As accelerators of bacterial infection and diffusion, TAAs can be used as effective weapons in the fight against bacterial infections.
New findings have been reviewed above. However, many questions remain. Firstly, an understanding of common adhesion mechanisms to cells or tissues, which is necessary for controlling bacterial infections, is urgently needed. Secondly, although many groups have studied the secretion mechanism, the precise details of the secretion process are required for a thorough understanding of TAA proteins. Thirdly, there may exist other receptors in addition to the molecules found on the cell surface or in tissues that promote bacterial invasion, which need to be determined. Meanwhile, the precise role of TAAs in pathogenesis should be given more attention. Finally, the activities of the protective sub-domains must be determined so that novel subunit vaccines can be developed. Therefore, though previous studies have been fruitful, much work remains to be done.
References
Abdullah M, Nepluev I, Afonina G, Ram S, Rice P, Cade W, Elkins C (2005) Killing of dsrA mutants of Haemophilus ducreyi by normal human serum occurs via the classical complement pathway and is initiated by immunoglobulin M binding. Infect Immun 73:3431–3439
Ackermann N, Tiller M, Anding G, Roggenkamp A, Heesemann J (2008) Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica. J Bacteriol 190(14):5031–5043 United States,2008
Aebi C, Lafontaine ER, Cope LD, Latimer JL, Lumbley SL, McCracken GH Jr, Hansen EJ (1998) Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun 66:3113–3119
Agnew C, Borodina E, Zaccai NR, Conners R, Burton NM, Vicary JA, Cole DK, Antognozzi M, Virji M, Brady RL (2011) Correlation of in situ mechanosensitive responses of the Moraxella catarrhalis adhesin UspA1 with fibronectin and receptor CEACAM1 binding. Proc Natl Acad Sci USA 108:15174–15178
Alvarez BH, Gruber M, Ursinus A, Dunin-Horkawicz S, Lupas AN, Zeth K (2010) A transition from strong right-handed to canonical left-handed supercoiling in a conserved coiled-coil segment of trimeric autotransporter adhesins. J Struct Biol 170:236–245
Attia AS, Ram S, Rice PA, Hansen EJ (2006) Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect Immun 74:1597–1611
Bernstein HD (2007) Are bacterial ‘autotransporters’ really transporters. Trends Microbiol 15:441–447
Biedzka-Sarek M, Salmenlinna S, Gruber M, Lupas AN, Meri S, Skurnik M (2008) Functional mapping of YadA- and Ail-mediated binding of human factor H to Yersinia enterocolitica serotype O:3. Infect Immun 76:5016–5027
Bolin I, Norlander L, Wolf-Watz H (1982) Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun 37:506–512
Bozza G, Capitani M, Montanari P, Benucci B, Biancucci M, Nardi-Dei V, Caproni E, Barrile R, Picciani B, Savino S, Arico B, Rappuoli R, Pizza M, Luini A, Sallese M, Merola M (2014) Role of ARF6, Rab11 and external Hsp90 in the trafficking and recycling of recombinant-soluble Neisseria meningitidis adhesin A (rNadA) in human epithelial cells. PLoS One 9:e110047
Cecchini P, Tavano R, de Laureto PP, Franzoso S, Mazzon C, Montanari P, Papini E (2011) The soluble recombinant Neisseria meningitidis adhesin NadA (Delta351-405) stimulates human monocytes by binding to extracellular Hsp90. PLoS One 6:e25089
Conners R, Hill DJ, Borodina E, Agnew C, Daniell SJ, Burton NM, Sessions RB, Clarke AR, Catto LE, Lammie D, Wess T, Brady RL, Virji M (2008) The Moraxella adhesin UspA1 binds to its human CEACAM1 receptor by a deformable trimeric coiled-coil. EMBO J 27:1779–1789
Cotter SE, Surana NK, St Geme JW 3rd (2005) Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol 13:199–205
Cotter SE, Surana NK, Grass S, St Geme JW 3rd (2006) Trimeric autotransporters require trimerization of the passenger domain for stability and adhesive activity. J Bacteriol 188:5400–5407
Cutter D, Mason KW, Howell AP, Fink DL, Green BA, St Geme JW 3rd (2002) Immunization with Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J Infect Dis 186:1115–1121
Daigneault MC, Lo RY (2009) Analysis of a collagen-binding trimeric autotransporter adhesin from Mannheimia haemolytica A1. FEMS Microbiol Lett 300:242–248
Dautin N, Bernstein HD (2007) Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol 61:89–112
Dautin N, Barnard TJ, Anderson DE, Bernstein HD (2007) Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J 26:1942–1952
Durant L, Metais A, Soulama-Mouze C, Genevard JM, Nassif X, Escaich S (2007) Identification of candidates for a subunit vaccine against extraintestinal pathogenic Escherichia coli. Infect Immun 75:1916–1925
Echenique-Rivera H, Brunelli B, Scarselli M, Taddei AR, Pizza M, Arico B, Serruto D (2011) A naturally occurring single-residue mutation in the translocator domain of Neisseria meningitidis NhhA affects trimerization, surface localization, and adhesive capabilities. Infect Immun 79:4308–4321
Edwards TE, Phan I, Abendroth J, Dieterich SH, Masoudi A, Guo W, Hewitt SN, Kelley A, Leibly D, Brittnacher MJ, Staker BL, Miller SI, Van Voorhis WC, Myler PJ, Stewart LJ (2010) Structure of a Burkholderia pseudomallei trimeric autotransporter adhesin head. PLoS One 5:e12803
Eitel J, Heise T, Thiesen U, Dersch P (2005) Cell invasion and IL-8 production pathways initiated by YadA of Yersinia pseudotuberculosis require common signalling molecules (FAK, c-Src, Ras) and distinct cell factors. Cell Microbiol 7:63–77
El-Kirat-Chatel S, Mil-Homens D, Beaussart A, Fialho AM, Dufrene YF (2013) Single-molecule atomic force microscopy unravels the binding mechanism of a Burkholderia cenocepacia trimeric autotransporter adhesin. Mol Microbiol 89:649–659
Fagnocchi L, Biolchi A, Ferlicca F, Boccadifuoco G, Brunelli B, Brier S, Norais N, Chiarot E, Bensi G, Kroll JS, Pizza M, Donnelly J, Giuliani MM, Delany I (2013) Transcriptional regulation of the nadA gene in Neisseria meningitidis impacts the prediction of coverage of a multicomponent meningococcal serogroup B vaccine. Infect Immun 81:560–569
Fusco WG, Choudhary NR, Routh PA, Ventevogel MS, Smith VA, Koch GG, Almond GW, Orndorff PE, Sempowski GD, Leduc I (2014) The Haemophilus ducreyi trimeric autotransporter adhesin DsrA protects against an experimental infection in the swine model of chancroid. Vaccine 32:3752–3758
Grin I, Hartmann MD, Sauer G, Hernandez AB, Schutz M, Wagner S, Madlung J, Macek B, Felipe-Lopez A, Hensel M, Lupas A, Linke D (2014) A trimeric lipoprotein assists in trimeric autotransporter biogenesis in enterobacteria. J Biol Chem 289:7388–7398
Grosskinsky U, Schutz M, Fritz M, Schmid Y, Lamparter MC, Szczesny P, Lupas AN, Autenrieth IB, Linke D (2007) A conserved glycine residue of trimeric autotransporter domains plays a key role in Yersinia adhesin A autotransport. J Bacteriol 189:9011–9019
Hallstrom T, Trajkovska E, Forsgren A, Riesbeck K (2006) Haemophilus influenzae surface fibrils contribute to serum resistance by interacting with vitronectin. J Immunol 177:430–436
Hartmann MD, Ridderbusch O, Zeth K, Albrecht R, Testa O, Woolfson DN, Sauer G, Dunin-Horkawicz S, Lupas AN, Alvarez BH (2009) A coiled-coil motif that sequesters ions to the hydrophobic core. Proc Natl Acad Sci USA 106:16950–16955
Hartmann MD, Grin I, Dunin-Horkawicz S, Deiss S, Linke D, Lupas AN, Hernandez AB (2012) Complete fiber structures of complex trimeric autotransporter adhesins conserved in enterobacteria. Proc Natl Acad Sci USA 109:20907–20912
Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D (2004) Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 68:692–744
Hill DJ, Edwards AM, Rowe HA, Virji M (2005) Carcinoembryonic antigen-related cell adhesion molecule (CEACAM)-binding recombinant polypeptide confers protection against infection by respiratory and urogenital pathogens. Mol Microbiol 55:1515–1527
Ishikawa M, Shigemori K, Hori K (2014) Application of the adhesive bacterionanofiber AtaA to a novel microbial immobilization method for the production of indigo as a model chemical. Biotechnol Bioeng 111:16–24
Jiang X, Ruiz T, Mintz KP (2011) The extended signal peptide of the trimeric autotransporter EmaA of Aggregatibacter actinomycetemcomitans modulates secretion. J Bacteriol 193:6983–6994
Jong W, Daleke-Schermerhorn MH, Vikstrom D, Ten HCM, de Punder K, van der Wel NN, van de Sandt CE, Rimmelzwaan GF, Follmann F, Agger E, Andersen P, de Gier JW, Luirink J (2014) An autotransporter display platform for the development of multivalent recombinant bacterial vector vaccines. Microb Cell Fact 13:162
Jose J (2006) Autodisplay: efficient bacterial surface display of recombinant proteins. Appl Microbiol Biotechnol 69:607–614
Junker M, Besingi RN, Clark PL (2009) Vectorial transport and folding of an autotransporter virulence protein during outer membrane secretion. Mol Microbiol 71:1323–1332
Kaiser PO, Riess T, Wagner CL, Linke D, Lupas AN, Schwarz H, Raddatz G, Schafer A, Kempf VA (2008) The head of Bartonella adhesin A is crucial for host cell interaction of Bartonella henselae. Cell Microbiol 10:2223–2234
Kaiser PO, Linke D, Schwarz H, Leo JC, Kempf VA (2012) Analysis of the BadA stalk from Bartonella henselae reveals domain-specific and domain-overlapping functions in the host cell infection process. Cell Microbiol 14:198–209
Kranen E, Detzel C, Weber T, Jose J (2014) Autodisplay for the co-expression of lipase and foldase on the surface of E. coli: washing with designer bugs. Microb Cell Fact 13:19
Laarmann S, Cutter D, Juehne T, Barenkamp SJ, St Geme JW (2002) The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Mol Microbiol 46:731–743
Leduc I, Olsen B, Elkins C (2009) Localization of the domains of the Haemophilus ducreyi trimeric autotransporter DsrA involved in serum resistance and binding to the extracellular matrix proteins fibronectin and vitronectin. Infect Immun 77:657–666
Lehr U, Schutz M, Oberhettinger P, Ruiz-Perez F, Donald JW, Palmer T, Linke D, Henderson IR, Autenrieth IB (2010) C-terminal amino acid residues of the trimeric autotransporter adhesin YadA of Yersinia enterocolitica are decisive for its recognition and assembly by BamA. Mol Microbiol 78:932–946
Leo JC, Elovaara H, Brodsky B, Skurnik M, Goldman A (2008) The Yersinia adhesin YadA binds to a collagenous triple-helical conformation but without sequence specificity. Protein Eng Des Sel 21:475–484
Leo JC, Lyskowski A, Hattula K, Hartmann MD, Schwarz H, Butcher SJ, Linke D, Lupas AN, Goldman A (2011) The structure of E. coli IgG-binding protein D suggests a general model for bending and binding in trimeric autotransporter adhesins. Structure 19:1021–1030
Linke D, Riess T, Autenrieth IB, Lupas A, Kempf VA (2006) Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol 14:264–270
Lu YY, Franz B, Truttmann MC, Riess T, Gay-Fraret J, Faustmann M, Kempf VA, Dehio C (2013) Bartonella henselae trimeric autotransporter adhesin BadA expression interferes with effector translocation by the VirB/D4 type IV secretion system. Cell Microbiol 15:759–778
Lyskowski A, Leo JC, Goldman A (2011) Structure and biology of trimeric autotransporter adhesins. Adv Exp Med Biol 715:143–158
Malito E, Biancucci M, Faleri A, Ferlenghi I, Scarselli M, Maruggi G, Lo SP, Veggi D, Liguori A, Santini L, Bertoldi I, Petracca R, Marchi S, Romagnoli G, Cartocci E, Vercellino I, Savino S, Spraggon G, Norais N, Pizza M, Rappuoli R, Masignani V, Bottomley MJ (2014) Structure of the meningococcal vaccine antigen NadA and epitope mapping of a bactericidal antibody. Proc Natl Acad Sci USA 111:17128–17133
Mazzon C, Baldani-Guerra B, Cecchini P, Kasic T, Viola A, de Bernard M, Arico B, Gerosa F, Papini E (2007) IFN-gamma and R-848 dependent activation of human monocyte-derived dendritic cells by Neisseria meningitidis adhesin A. J Immunol 179:3904–3916
Meng G, Surana NK, St Geme JW 3rd, Waksman G (2006) Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter. EMBO J 25:2297–2304
Meng G, St Geme JW 3rd, Waksman G (2008) Repetitive architecture of the Haemophilus influenzae Hia trimeric autotransporter. J Mol Biol 384:824–836
Mil-Homens D, Fialho AM (2011) Trimeric autotransporter adhesins in members of the Burkholderia cepacia complex: a multifunctional family of proteins implicated in virulence. Front Cell Infect Microbiol 1:13
Mintz KP (2004) Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology 150:2677–2688
Montanari P, Bozza G, Capecchi B, Caproni E, Barrile R, Norais N, Capitani M, Sallese M, Cecchini P, Ciucchi L, Gao Z, Rappuoli R, Pizza M, Arico B, Merola M (2012) Human heat shock protein (Hsp) 90 interferes with Neisseria meningitidis adhesin A (NadA)-mediated adhesion and invasion. Cell Microbiol 14:368–385
Muller NF, Kaiser PO, Linke D, Schwarz H, Riess T, Schafer A, Eble JA, Kempf VA (2011) Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infect Immun 79:2544–2553
Nordstrom T, Blom AM, Forsgren A, Riesbeck K (2004) The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J Immunol 173:4598–4606
Nummelin H, Merckel MC, el Tahir Y, Ollikka P, Skurnik M, Goldman A (2003) Structural studies of Yersinia adhesin YadA. Adv Exp Med Biol 529:85–88
Nummelin H, Merckel MC, Leo JC, Lankinen H, Skurnik M, Goldman A (2004) The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel beta-roll. EMBO J 23:701–711
Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P (2004) Structure of the translocator domain of a bacterial autotransporter. EMBO J 23:1257–1266
Paczosa MK, Fisher ML, Maldonado-Arocho FJ, Mecsas J (2014) Yersinia pseudotuberculosis uses Ail and YadA to circumvent neutrophils by directing Yop translocation during lung infection. Cell Microbiol 16:247–268
Pantophlet R, Burton DR (2003) Immunofocusing: antigen engineering to promote the induction of HIV-neutralizing antibodies. Trends Mol Med 9:468–473
Peterson JH, Tian P, Ieva R, Dautin N, Bernstein HD (2010) Secretion of a bacterial virulence factor is driven by the folding of a C-terminal segment. Proc Natl Acad Sci USA 107:17739–17744
Radin JN, Grass SA, Meng G, Cotter SE, Waksman G, St Geme JW 3rd (2009) Structural basis for the differential binding affinities of the HsfBD1 and HsfBD2 domains in the Haemophilus influenzae Hsf adhesin. J Bacteriol 191:5068–5075
Raghunathan D, Wells TJ, Morris FC, Shaw RK, Bobat S, Peters SE, Paterson GK, Jensen KT, Leyton DL, Blair JM, Browning DF, Pravin J, Flores-Langarica A, Hitchcock JR, Moraes CT, Piazza RM, Maskell DJ, Webber MA, May RC, MacLennan CA, Piddock LJ, Cunningham AF, Henderson IR (2011) SadA, a trimeric autotransporter from Salmonella enterica serovar Typhimurium, can promote biofilm formation and provides limited protection against infection. Infect Immun 79:4342–4352
Riess T, Andersson SG, Lupas A, Schaller M, Schafer A, Kyme P, Martin J, Walzlein JH, Ehehalt U, Lindroos H, Schirle M, Nordheim A, Autenrieth IB, Kempf VA (2004) Bartonella adhesin a mediates a proangiogenic host cell response. J Exp Med 200:1267–1278
Roggenkamp A, Ackermann N, Jacobi CA, Truelzsch K, Hoffmann H, Heesemann J (2003) Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J Bacteriol 185:3735–3744
Schmid Y, Grassl GA, Buhler OT, Skurnik M, Autenrieth IB, Bohn E (2004) Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells. Infect Immun 72:6780–6789
Schutz M, Weiss EM, Schindler M, Hallstrom T, Zipfel PF, Linke D, Autenrieth IB (2010) Trimer stability of YadA is critical for virulence of Yersinia enterocolitica. Infect Immun 78:2677–2690
Serruto D, Spadafina T, Scarselli M, Bambini S, Comanducci M, Hohle S, Kilian M, Veiga E, Cossart P, Oggioni MR, Savino S, Ferlenghi I, Taddei AR, Rappuoli R, Pizza M, Masignani V, Arico B (2009) HadA is an atypical new multifunctional trimeric coiled-coil adhesin of Haemophilus influenzae biogroup aegyptius, which promotes entry into host cells. Cell Microbiol 11:1044–1063
Shahid SA, Markovic S, Linke D, van Rossum BJ (2012) Assignment and secondary structure of the YadA membrane protein by solid-state MAS NMR. Sci Rep 2:803
Sheets AJ, St Geme JW 3rd (2011) Adhesive activity of the haemophilus cryptic genospecies cha autotransporter is modulated by variation in tandem peptide repeats. J Bacteriol 193:329–339
Sheets AJ, Grass SA, Miller SE, St Geme JW 3rd (2008) Identification of a novel trimeric autotransporter adhesin in the cryptic genospecies of Haemophilus. J Bacteriol 190:4313–4320
Singh B, Jubair TA, Morgelin M, Sundin A, Linse S, Nilsson UJ, Riesbeck K (2014a) Haemophilus influenzae surface fibril (Hsf) is a unique twisted hairpin-like trimeric autotransporter. Int J Med Microbiol 305:27–37
Singh B, Su YC, Al-Jubair T, Mukherjee O, Hallstrom T, Morgelin M, Blom AM, Riesbeck K (2014b) A fine-tuned interaction between trimeric autotransporter haemophilus surface fibrils and vitronectin leads to serum resistance and adherence to respiratory epithelial cells. Infect Immun 82:2378–2389
Sjolinder H, Eriksson J, Maudsdotter L, Aro H, Jonsson AB (2008) Meningococcal outer membrane protein NhhA is essential for colonization and disease by preventing phagocytosis and complement attack. Infect Immun 76:5412–5420
Skurnik M, Bolin I, Heikkinen H, Piha S, Wolf-Watz H (1984) Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol 158:1033–1036
Spahich NA, St Geme JW 3rd (2011) Structure and function of the Haemophilus influenzae autotransporters. Front Cell Infect Microbiol 1:5
Surana NK, Cutter D, Barenkamp SJ, St Geme JW 3rd (2004) The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J Biol Chem 279:14679–14685
Szabady RL, Peterson JH, Skillman KM, Bernstein HD (2005) An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc Natl Acad Sci USA 102:221–226
Szczesny P, Lupas A (2008) Domain annotation of trimeric autotransporter adhesins–daTAA. Bioinformatics 24:1251–1256
Szczesny P, Linke D, Ursinus A, Bar K, Schwarz H, Riess TM, Kempf VA, Lupas AN, Martin J, Zeth K (2008) Structure of the head of the Bartonella adhesin BadA. PLoS Pathog 4:e1000119
Tahir YE, Kuusela P, Skurnik M (2000) Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification Of eight NSVAIG—S motifs in the amino-terminal half of the protein involved in collagen binding. Mol Microbiol 37:192–206
Tang G, Ruiz T, Mintz KP (2012) O-polysaccharide glycosylation is required for stability and function of the collagen adhesin EmaA of Aggregatibacter actinomycetemcomitans. Infect Immun 80:2868–2877
Tavano R, Capecchi B, Montanari P, Franzoso S, Marin O, Sztukowska M, Cecchini P, Segat D, Scarselli M, Arico B, Papini E (2011) Mapping of the Neisseria meningitidis NadA cell-binding site: relevance of predicted {alpha}-helices in the NH2-terminal and dimeric coiled-coil regions. J Bacteriol 193:107–115
Tiyawisutsri R, Holden MT, Tumapa S, Rengpipat S, Clarke SR, Foster SJ, Nierman WC, Day NP, Peacock SJ (2007) Burkholderia Hep_Hag autotransporter (BuHA) proteins elicit a strong antibody response during experimental glanders but not human melioidosis. BMC Microbiol 7:19
Totsika M, Wells TJ, Beloin C, Valle J, Allsopp LP, King NP, Ghigo JM, Schembri MA (2012) Molecular characterization of the EhaG and UpaG trimeric autotransporter proteins from pathogenic Escherichia coli. Appl Environ Microbiol 78:2179–2189
Ulrich T, Oberhettinger P, Schutz M, Holzer K, Ramms AS, Linke D, Autenrieth IB, Rapaport D (2014) Evolutionary conservation in biogenesis of beta-barrel proteins allows mitochondria to assemble a functional bacterial trimeric autotransporter protein. J Biol Chem 289:29457–29470
Veiga E, de Lorenzo V, Fernandez LA (1999) Probing secretion and translocation of a beta-autotransporter using a reporter single-chain Fv as a cognate passenger domain. Mol Microbiol 33:1232–1243
Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J (2003) Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265
Wang L, Qin W, Ruidong Z, Liu S, Zhang H, Sun C, Feng X, Gu J, Du C, Han W, Langford PR, Lei L (2014a) Differential gene expression profiling of Actinobacillus pleuropneumoniae during induction of primary alveolar macrophage apoptosis in piglets. Microb Pathog 78C:74–86
Wang YP, Hsieh MK, Tan DH, Shien JH, Ou SC, Chen CF, Chang PC (2014b) The haemagglutinin of Avibacterium paragallinarum is a trimeric autotransporter adhesin that confers haemagglutination, cell adherence and biofilm formation activities. Vet Microbiol 174:474–482
Wang L, Qin W, Ruidong Z, Liu S, Zhang H, Sun C, Feng X, Gu J, Du C, Han W, Langford PR, Lei L (2015a) Differential gene expression profiling of Actinobacillus pleuropneumoniae during induction of primary alveolar macrophage apoptosis in piglets. Microb Pathog 78:74–86
Wang L, Qin W, Yang S, Zhai R, Zhou L, Sun C, Pan F, Ji Q, Wang Y, Gu J, Feng X, Du C, Han W, Langford PR, Lei L (2015b) The Adh adhesin domain is required for trimeric autotransporter Apa1-mediated Actinobacillus pleuropneumoniae adhesion, autoaggregation, biofilm formation and pathogenicity. Vet Microbiol 177:175–183
Wells TJ, Tree JJ, Ulett GC, Schembri MA (2007) Autotransporter proteins: novel targets at the bacterial cell surface. FEMS Microbiol Lett 274:163–172
Weynants VE, Feron CM, Goraj KK, Bos MP, Denoel PA, Verlant VG, Tommassen J, Peak IR, Judd RC, Jennings MP, Poolman JT (2007) Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun 75:5434–5442
Xiao L, Zhou L, Sun C, Feng X, Du C, Gao Y, Ji Q, Yang S, Wang Y, Han W, Langford PR, Lei L (2012) Apa is a trimeric autotransporter adhesin of Actinobacillus pleuropneumoniae responsible for autoagglutination and host cell adherence. J Basic Microbiol 52:598–607
Yeo HJ, Cotter SE, Laarmann S, Juehne T, St Geme JW 3rd, Waksman G (2004) Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J 23:1245–1256
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 31,372,447) and by Specialized Research Fund for the Doctoral Program of Higher Education (NO.20130061110089).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lei Wang—Co-first author
Rights and permissions
About this article
Cite this article
Qin, W., Wang, L. & Lei, L. New findings on the function and potential applications of the trimeric autotransporter adhesin. Antonie van Leeuwenhoek 108, 1–14 (2015). https://doi.org/10.1007/s10482-015-0477-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0477-4