Abstract
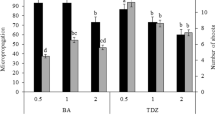
This report describes a protocol for the in vitro shoot induction and plant regeneration from epicotyl explants of Cassia angustifolia on Murashige and Skoog (MS) medium supplemented with 6-benzyladenine (BA), Kinetin and 2-iP (0.5–10.0 μM). MS medium supplemented with BA (5.0 μM) was the most effective in inducing adventitious shoots and growth. The highest rate of shoot multiplication was achieved on MS medium supplemented with BA (5.0 μM) and Indole-3-acetic acid (IAA, 1.0 μM). The nodal segments excised from the shoots regenerated from BA (5.0 μM) and IAA (1.0 μM) were used as explants for next three round of micropropagation. The number of shoots significantly increased at successive round of micropropagation. For rooting, MS medium supplemented with 2.0 μM indole-3-butyric acid proved to be better than that supplemented with IAA or α-naphthalene acetic acid. The in vitro raised plantlets with well developed shoot and roots were successfully established in earthen pots containing garden soil and were grown in greenhouse. About 52 plants (85 %) survived out of 60 plants transferred in garden soil.



Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- Kin:
-
Kinetin
- 2-iP:
-
2-Isopentenyl adenine
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
α-Naphthalene acetic acid
- PGRs:
-
Plant growth regulators
References
Agrawal V, Sardar PR (2003) In vitro organogenesis and histomorphological investigations in Senna (Cassia angustifolia) a medicinally valuable shrub. Physiol Mol Biol Plants 91:131–140
Agrawal V, Sardar PR (2006) In vitro propagation of Cassia angustifolia (Vahl.) through leaflet and cotyledon derived calli. Biol Plant 1:118–122
Agrawal V, Sardar PR (2007) In vitro regeneration through somatic embryogenesis and organogenesis using cotyledons of Cassia angustifolia Vahl. In Vitro Cell Dev Biol Plant 43:585–592
Ahmad N, Anis M (2011) An efficient in vitro process for recurrent production of cloned plants of Vitex negundo L. Eur J For Res 130:135–144
Ahmad N, Faisal M, Anis M, Aref IM (2010) In vitro callus induction and plant regeneration from leaf explants of Ruta graveolens L. South Afr J Bot 76:597–600
Anonymous (1992) The wealth of India: a dictionary of Indian raw materials and industrial products, vol 3. CSIR, New Delhi, pp 354–363
Bohra NK, Sankhla PS (1997) Senna—a cash crop for arid regions. Vaniki Sandesh 21:19–23
Chen CU, Hsia CN, Yeh MS, Agrawal DC, Tsay HS (2006) In vitro micropropagation and ex vitro acclimation of Bupleurum kaoi: an endangered medicinal plant native to Taiwan. In Vitro Cell Dev Biol Plant 42:128–133
Dhavala A, Rathore TS (2010) Micropropagation of Embelia ribes Burm f. through proliferation of adult plant axillary shoots. In Vitro Cell Dev Biol Plant 46:180–191
Escalettes V, Françoise D (1993) In vitro adventitious shoot regeneration from leaves of Prunus spp. Plant Sci 90:201–209
Faisal M, Anis M (2003) Rapid mass propagation of Tylophora indica Merill via leaf callus culture. Plant Cell Tissue Organ Cult 75:125–129
Faisal M, Ahmad N, Anis M (2005) Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult 80:187–190
Franz G (1993) The senna drug and its chemistry. Pharmacology 47:2–6
Hung CD, Johnson K, Torpy F (2006) Liquid culture for efficient micropropagation of Wasabia japonica (MIQ.) Matsumura. In Vitro Cell Dev Biol Plant 42:548–552
Jain N, Babbar SB (2000) Recurrent production of plants of black plum, Syzgium cumini (L.) Skeels, a myrtaceous fruit tree from in vitro cultured seedling explants. Plant Cell Rep 19:519–524
Khalafalla MM, Daffalla HM, Abdellatef E, Agabna E, El-Shemy HA (2011) Establishment of an in vitro micropropagation protocol for Boscia senegalensis (Pers.) Lam. ex Poir. J Zhejiang Univ Sci B 12:303–312
Koroch K, Juliani HP, Kapteyn J, Simon JE (2003) In vitro regeneration of Echinacea purpurea from leaf explants. Plant Cell Tissue Organ Cult 69:79–83
Maharik N, Elgengaini S, Taha H (2009) In vitro mass propagation of the endangered Sinai hawthorn Crataegus sinaica Boiss. Int J Acad Res 1:24–29
Mongomake K, Hilaire KT, Daouda K, Michel Z, Justin KY, Ochatt SJ (2009) In vitro plantlets regeneration in Bambara groundnut [Vigna subterranean (L.) Verdc. (Fabaceae)] through direct shoot bud differentiation on hypocotyl and epicotyls cuttings. African J Biotech 8:1466–1473
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Oliveira YD, Pinto F, Lopes da Silva AL, Guedes I, Biasi LA, Quoirin M (2010) An efficient protocol for micropropagation of Melaleuca alternifolia Cheel. In Vitro Cell Dev Biol Plant 46:192–197
Pence VC (1999) The application of biotechnology for the conservation of endangered plants. In: Benson EE (ed) Plant conservation biotechnology, vol 15. Taylor and Francis, London, pp 227–241
Popelka JC, Gollasch S, Moore A, Molvig L, Higgins TJV (2006) Genetic transformation of cowpea (Vigna unguiculata L.) and stable transmission of the transgenes to progeny. Plant Cell Rep 25:304–312
Rout GR (2004) Effect of cytokinins and auxins on micropropagation of Clitorea ternatea L. Biol Lett 41:21–26
Rout GR, Reddy GM, Das P (2001) Studies on in vitro clonal propagation of Paulownia tomentosa Steud, and evaluation of genetic fidelity through RAPD marker. Silvae Genet 50:208–212
Shailendra V, Joshi N, Tak K, Purohit SD (2005) In vitro adventitious shoot bud differentiation and plantlet regeneration in Feronia limonia L. (swingle). In Vitro Cell Dev Biol Plant 41:296–302
Sharma AK, Goyal RK, Gupta JP (1999) Senna the best choice for sandy wastelands. Indian Farming 6:18–20
Siddique I, Anis M (2007a) High frequency multiple shoot regeneration and plantlet formation in Cassia angustifolia (Vahl.) using thidiazuron. Med Arom Plant Sci Biotech 1:282–284
Siddique I, Anis M (2007b) In vitro shoot multiplication and plant regeneration from nodal explants of Cassia angustifolia (Vahl.): a medicinal plant. Acta Physiol Plant 29:233–238
Siddique I, Anis M (2008) An improved plant regeneration system and ex vitro acclimatization of Ocimum basilicum L. Acta Physiol Plant 30:493–499
Siddique I, Anis M, Aref IM (2010) In vitro adventitious shoot regeneration via indirect organogenesis from petiole explants of Cassia angustifolia Vahl. a potential medicinal plant. Appl Biochem Biotechnol 162:2067–2074
Somera DA, Samac DA, Olhoft PM (2003) Recent advances in legume transformation. Plant Physiol 131:892–899
Tsay HS, Gau TG, Chen CC (1989) Rapid clonal propagation of Pinellia ternata by tissue culture. Plant Cell Rep 8:450–454
Acknowledgments
This research project was supported by a grant from the research centre of the centre for female scientific and medical colleges in King Saud University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siddique, I., Javed, S.B., Al-Othman, M.R. et al. Stimulation of in vitro organogenesis from epicotyl explants and successive micropropagation round in Cassia angustifolia Vahl.: an important source of sennosides. Agroforest Syst 87, 583–590 (2013). https://doi.org/10.1007/s10457-012-9578-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-012-9578-5