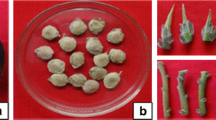
Abstract
An efficient in vitro process for rapid clonal propagation of a 40-year-old tree of Azadirachta indica employing nodal stem segments was developed. Season of collection and maturity of explants showed direct influence on bud-break. Nodal stem segments collected during the month of April gave best response. Maximum bud-break (78.6–81%) was obtained when middle order nodes (3rd or 4th node from apex) were taken. Amongst different cytokinins used, 6-benzylaminopurine (BAP) at the concentration of 1.11 μM was found most effective in inducing multiple shoots, whereas inorganic and organic constituents of the medium influenced growth and general condition of proliferating shoots. On an average 3.1 shoots per explant were regenerated in modified Murashige and Skoog medium supplemented with 1.11 μM BAP, 1.43 μM indole-3-acetic acid (IAA) and 81.43 μM adenine hemisulphate. Isolated shoots were rooted in presence of 2.46 μM indole-3-butryic acid (IBA). Root induction took place in 8–10 days with 100% rooting. The in vitro-raised plantlets were successfully transplanted in potted soil and finally grown under field conditions with 100% survival. The genetic fidelity of such in vitro-raised field-grown plants was ascertained by random amplified polymorphic DNA (RAPD) markers. Furthermore, the azadirachtin content of in vitro-cloned plants was found comparable to the mother tree proving their chemical stability also. The protocol developed holds good for in vitro cloning of mature elite neem trees.

Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzylaminopurine
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- 2iP:
-
N 6-(2-isopentenyl) adenine
- MS medium:
-
Murashige and Skoog medium
- NAA:
-
α-Naphthaleneacetic acid
- PCR:
-
Polymerase chain reaction
- PVP:
-
Polyvinylpyrollidone
- RAPD:
-
Random amplified polymorphic DNA
- Taq :
-
Thermus aquaticus
- TDZ:
-
Thidiazuron or (N-phenyl-N′-(1,2,3-thiadiazol-yl) urea
- Z:
-
Zeatin
References
Ajithkumar D, Seeni S (1998) Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep 17:422–426. doi:10.1007/s002990050418
Arya S, Rathore TS, Arya ID (1995) In vitro propagation of neem from seedling and mature tree. Indian J Plant Genet Resour 8:247–252
Bhatt ID, Dhar U (2004) Factors controlling micropropagation of Myrica esculenta buch.—Ham. ex D. Don: a high value wild edible of Kumaun Himalaya. Afr J Biotechnol 3:534–540
Chand S, Singh AK (2004) In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree, Pterocarpus marsupium Roxb. In Vitro Cell Dev Biol Plant 40:167–170. doi:10.1079/IVP2003488
Chaturvedi R, Razdan MK, Bhojwani SS (2004) In vitro clonal propagation of an adult tree of neem (Azadirachta indica A. Juss.) by forced axillary branching. Plant Sci 166:501–506. doi:10.1016/j.plantsci.2003.10.021
de Kochko A, Hamon S (1990) A rapid and efficient method for the isolation of restrictable total DNA from plants of genus Abelmoschus. Plant Mol Biol Rep 8:3–7. doi:10.1007/BF02668874
Dhar U, Upreti J (1999) In vitro regeneration of a mature leguminous liana (Bauhinia vahlii Wight and Arnott). Plant Cell Rep 18:664–669. doi:10.1007/s002990050639
Eeswara JP, Stuchbury T, Allan EJ, Mordue (Luntz) AJ (1998) A standard procedure for the micropropagation of the neem tree (Azadirachta indica A. Juss.). Plant Cell Rep 17:215–219. doi:10.1007/s002990050381
Elmore HW, Samples B, Sharma S, Harrison M (1990) Influence of cultural and physiochemical factors on ascorbate stability in plant tissue culture media. Plant Cell Tissue Organ Cult 20:131–135. doi:10.1007/BF00114711
Ermel K, Pahlich E, Schmutterer H (1987) Azadirachtin content of neem kernels from different geographical locations and its dependence on temperature, relative humidity and light. In: Schmutterer H, Ascher KRS (eds) Natural pesticides from the neem tree (Azadirachta indica A. Juss.) and other tropical plants. Proc Third Intl Neem Conference, Nairobi. German Agency for Technical Cooperation, Eschborn, pp 171–184
Farooqui N, Ranade SA, Sane PV (1998) RAPD profile variation amongst provenances of neem. Biochem Mol Biol Int 45:931–939
Gangopadhyay G, Bandyopadhyay T, Modak BK, Wongpornchai S, Mukherjee KK (2004) Micropropagation of Indian pandan (Pandanus amaryllifolius Roxb.), a rich source of 2-acetyl-1-pyrroline. Curr Sci 87:1589–1592
Gautam VK, Nanda K, Gupta SC (1993) Development of shoots and roots in anther-derived callus of Azadirachta indica A. Juss.—a medicinal tree. Plant Cell Tissue Organ Cult 34:13–18. doi:10.1007/BF00048458
George EF, Sherrington PD (1984) Plant propagation by tissue culture, handbook and directory of commercial laboratories. Exegetics, England
Hsia CN, Korban SS (1996) Factors affecting in vitro establishment and shoot proliferation of Rosa hybrida L. and Rosa chinensis minima. In Vitro Cell Dev Biol Plant 32:217–222. doi:10.1007/BF02822690
Islam R, Hoque A, Khalekuzzaman M, Joarder OI (1997) Micropropagation of Azadirachta indica A. Juss from explants of mature plants. Plant Tissue Cult 7:41–46
Johnson M, Manickam VS (2003) In vitro micropropagation of Baliospermum montanum (Willd.) Muell-Arg—a medicinal plant. Indian J Exp Biol 41:1349–1351
Joshi MS, Thengane SR (1996) In vitro propagation of Azadirachta indica A. Juss. (neem) by shoot proliferation. Indian J Exp Biol 34:480–482
Kaur K, Verma B, Kant U (1998) Plants obtained from the Khair tree (Acacia catechu Willd.) using mature nodal segments. Plant Cell Rep 17:427–429. doi:10.1007/s002990050419
Knop W (1865) Quantitative Utersuchungen über den Ernährungsprocess der Pflanze. Landw Versuchs-Stat 7:93–107
Kumar R, Sharma K, Agrawal V (2005) In vitro clonal propagation of Holarrhena antidysenterica (L.) Wall. through nodal explants from mature trees. In Vitro Cell Dev Biol Plant 41:137–144. doi:10.1079/IVP2004624
Mathur N, Ramawat KG, Nandwani D (1995) Rapid in vitro multiplication of jujube through mature stem explants. Plant Cell Tissue Organ Cult 43:75–77. doi:10.1007/BF00042675
Misra P, Chaturvedi HC (1991) Influence of inorganic salts on cytokinin induced caulogenesis in leaf segments of Rosmarinus officinalis L. Plant Sci 79:229–235. doi:10.1016/0168-9452(91)90110-T
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nanda RM, Das P, Rout GR (2004) In vitro clonal propagation of Acacia mangium Willd. and its evaluation of genetic stability through RAPD marker. Ann Sci 61:381–386. doi:10.1051/forest:2004031
Ndoye M, Diallo I, Gassama YK (2003) In vitro multiplication of the semi-arid forest tree, Balanites aegyptiaca (L.) Del. Afr J Biotechnol 2:421–424
Quraishi A, Koche V, Sharma P, Mishra SK (2004) In vitro clonal propagation of neem (Azadirachta indica). Plant Cell Tissue Organ Cult 78:281–284. doi:10.1023/B:TICU.0000025647.58548.3d
Rout GR, Das P, Goel S, Raina SN (1998) Determination of genetic stability of micropropagated plants of ginger using random amplified polymorphic DNA (RAPD) markers. Bot Bull Acad Sin 39:23–27
Rout GR, Reddy GM, Das P (2001) Studies on in vitro clonal propagation of Paulownia tomentosa STEUD and evaluation of genetic fidelity through RAPD marker. Silvae Genet 50:208–212
Salvi ND, Hemraj S, Suchita T, Eapen S (2001) Plant regeneration from different explants of neem. Plant Cell Tissue Organ Cult 65:159–162. doi:10.1023/A:1010672809141
Schmutterer H (ed) (1995) The neem tree Azadirachta indica A. Juss. and other meliaceous plants—sources of unique natural products for integrated pest management, medicine, industry and other purposes. VCH, Weinheim, Germany
Sharma PK, Tyagi P, Sharma KC, Kothari SL (2003) Clonal micropropagation of Crataeva adansonii (DC.) Prodr.: a multipurpose tree. In Vitro Cell Dev Biol Plant 39:156–160. doi:10.1079/IVP2002384
Sidhu OP, Behl HM (1996) Seasonal variation in azadirachtins in seeds of Azadirachta indica. Curr Sci 70:1084–1086
Singh A, Negi MS, Moses VK, Venkateswarlu B, Srivastava PS, Lakshmikumaran M (2002) Molecular analysis of micropropagated neem plants using AFLP markers for ascertaining clonal fidelity. In Vitro Cell Dev Biol Plant 38:519–524. doi:10.1079/IVP2002341
Siril EA, Dhar U (1997) Micropropagation of mature Chinese tallow tree (Sapium sebiferum Roxb.). Plant Cell Rep 16:637–640. doi:10.1007/BF01275506
Sudhersan C, Hussain J (2003) In vitro clonal propagation of a multipurpose tree, Ziziphus spina-christi (L.) Desf. Turk J Bot 27:167–171
Venkateshwarlu B, Katyal JC, Choudhuri J, Mukhopadhyay K (1998) Micropropagation of plus neem (Azadirachta indica A Juss) and evaluation of field transferred plants. Indian For 124:537–543
Venkateswarlu B, Mukhopadhyaya J (1999) Azadirachtin content in the seeds of micropropagated neem plants in relation to its mother tree. Curr Sci 76:626–627
Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535. doi:10.1093/nar/18.22.6531
Acknowledgments
The authors thank the Director, National Botanical Research Institute (NBRI), Lucknow for the facilities provided. The first author also thanks the Council of Scientific and Industrial Research (CSIR), India, for awarding her Senior Research Fellowship. Thanks are also due to Dr. H. M. Behl and Dr. Vishal Kumar for providing facilities and guidance for work related to chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arora, K., Sharma, M., Srivastava, J. et al. Rapid in vitro cloning of a 40-year-old tree of Azadirachta indica A. Juss. (Neem) employing nodal stem segments. Agroforest Syst 78, 53–63 (2010). https://doi.org/10.1007/s10457-009-9230-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-009-9230-1