Abstract
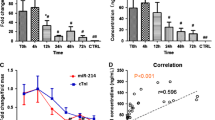
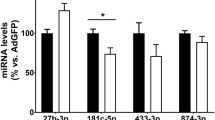
Peripheral artery disease (PAD) is an occlusive disease of limb arteries. Critical limb ischemia (CLI) is an advanced form of PAD that is prognostically worse in subjects with diabetes and can result in limb loss, gangrene, and death, although the underlying signaling mechanisms that contribute to its development remain poorly understood. By comparing plasma samples from diabetic humans with PAD and mouse models of PAD, we identified miR-375 to be significantly downregulated in humans and mice during progression to CLI. Overexpression of miR-375 was pro-angiogenic in endothelial cells in vitro and induced endothelial migration, proliferation, sprouting, and vascular network formation, whereas miR-375 inhibition conferred anti-angiogenic effects. Intramuscular delivery of miR-375 improved blood flow recovery to diabetic mouse hindlimbs following femoral artery ligation (FAL) and improved neovessel growth and arteriogenesis in muscle tissues. Using RNA-sequencing and prediction algorithms, Kruppel-like factor 5 (KLF5) was identified as a direct target of miR-375 and siRNA knockdown of KLF5 phenocopied the effects of miR-375 overexpression in vitro and in vivo through regulatory changes in NF-kB signaling. Together, a miR-375-KLF5-NF-kB signaling axis figures prominently as a potential therapeutic pathway in the development CLI in diabetes.










Similar content being viewed by others
Data availability
All relevant data are available from the authors. The RNA-seq data are accessible at: GSE210778. Source data are provided with this paper.
Abbreviations
- 3′UTR:
-
3′ untranslated region
- ALI:
-
Acute limb ischemia
- CAECS:
-
Coronary artery endothelial cells
- CASMCs:
-
Coronary artery smooth muscle cells
- CD31:
-
Cluster of differentiation 31; PECAM
- ChIP:
-
Chromatin immunoprecipitation
- CLI:
-
Critical limb ischemia
- Db/+:
-
Heterozygous leptin receptor deficient, non-diabetic
- Db/Db:
-
Homozygous leptin receptor deficient, diabetic
- EC:
-
Endothelial cell
- FAL:
-
Femoral artery ligation
- HAECs:
-
Human aortic endothelial cells
- HIF1α:
-
Hypoxia-inducible factor alpha
- HLI:
-
Hind limb ischemia
- HUVECs:
-
Human umbilical vein endothelial cells
- IKKB:
-
Inhibitor of Nuclear factor kappa-B kinase subunit
- Beta IL-1β:
-
Interleukin-1 Beta
- IL-8:
-
Interleukin-8
- IPA:
-
Ingenuity pathway analysis
- IkBα:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- KLF4:
-
Kruppel-like factor 4
- KLF5:
-
Kruppel-like factor 5
- LPS:
-
Lipopolysaccharide
- miRNA:
-
microRNA
- miR-375:
-
microRNA-375-3p
- MOVAS:
-
Mouse vascular smooth muscle cells
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Non-EC:
-
Non-endothelial cell
- p65:
-
Nuclear factor NF-kappa-B p65 subunit
- PAD:
-
Peripheral artery disease
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- siRNA:
-
Short Interfering RNA
- SLI:
-
Sub-acute limb ischemia
- TNFα:
-
Tumor necrosis factor alpha
- αSMA:
-
Smooth muscle actin alpha
References
Criqui MH (2001) Systemic atherosclerosis risk and the mandate for intervention in atherosclerotic peripheral arterial disease. Am J Cardiol 88(7B):43J–47J. https://doi.org/10.1016/s0002-9149(01)01881-1
Hardman RL, Jazaeri O, Yi J, Smith M, Gupta R (2014) Overview of classification systems in peripheral artery disease. Semin Intervent Radiol 31(4):378–388. https://doi.org/10.1055/s-0034-1393976
Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH (2013) Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 382(9901):1329–1340. https://doi.org/10.1016/S0140-6736(13)61249-0
Marso SP, Hiatt WR (2006) Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 47(5):921–929. https://doi.org/10.1016/j.jacc.2005.09.065
Bonaca MP, Creager MA (2015) Pharmacological treatment and current management of peripheral artery disease. Circ Res 116(9):1579–1598. https://doi.org/10.1161/CIRCRESAHA.114.303505
Cooke JP, Losordo DW (2015) Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res 116(9):1561–1578. https://doi.org/10.1161/CIRCRESAHA.115.303565
Beach JM (2021) Revascularization strategies for acute and chronic limb ischemia. Cardiol Clin 39(4):483–494. https://doi.org/10.1016/j.ccl.2021.06.006
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233. https://doi.org/10.1016/j.cell.2009.01.002
Icli B, Wara AK, Moslehi J, Sun X, Plovie E, Cahill M, Marchini JF, Schissler A, Padera RF, Shi J, Cheng HW, Raghuram S, Arany Z, Liao R, Croce K, MacRae C, Feinberg MW (2013) MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res 113(11):1231–1241. https://doi.org/10.1161/CIRCRESAHA.113.301780
Icli B, Wu W, Ozdemir D, Li H, Cheng HS, Haemmig S, Liu X, Giatsidis G, Avci SN, Lee N, Guimaraes RB, Manica A, Marchini JF, Rynning SE, Risnes I, Hollan I, Croce K, Yang X, Orgill DP, Feinberg MW (2019) MicroRNA-615-5p regulates angiogenesis and tissue repair by targeting AKT/eNOS (protein kinase b/endothelial nitric oxide synthase) signaling in endothelial cells. Arterioscler Thromb Vasc Biol 39(7):1458–1474. https://doi.org/10.1161/ATVBAHA.119.312726
Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L (2015) TGFbeta triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res 116(11):1753–1764. https://doi.org/10.1161/CIRCRESAHA.116.305178
Liang YZ, Li JJ, Xiao HB, He Y, Zhang L, Yan YX (2020) Identification of stress-related microRNA biomarkers in type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes 12(9):633–644. https://doi.org/10.1111/1753-0407.12643
Zhou H, Peng C, Huang DS, Liu L, Guan P (2020) microRNA expression profiling based on microarray approach in human diabetic retinopathy: a systematic review and meta-analysis. DNA Cell Biol 39(3):441–450. https://doi.org/10.1089/dna.2019.4942
Perez-Cremades D, Cheng HS, Feinberg MW (2020) Noncoding RNAs in critical limb ischemia. Arterioscler Thromb Vasc Biol 40(3):523–533. https://doi.org/10.1161/ATVBAHA.119.312860
Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA, Committee TPTS, Investigators (2012) Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 366(15):1404–1413. https://doi.org/10.1056/NEJMoa1200933
Vlachos IS, Paraskevopoulou MD, Karagkouni D, Georgakilas G, Vergoulis T, Kanellos I, Anastasopoulos IL, Maniou S, Karathanou K, Kalfakakou D, Fevgas A, Dalamagas T, Hatzigeorgiou AG (2015) DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res 43 (Database issue):D153–159. https://doi.org/10.1093/nar/gku1215
McGeary SE, Lin KS, Shi CY, Pham TM, Bisaria N, Kelley GM, Bartel DP (2019) The biochemical basis of microRNA targeting efficacy. Science. https://doi.org/10.1126/science.aav1741
Chen Y, Wang X (2020) miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res 48(D1):D127–D131. https://doi.org/10.1093/nar/gkz757
Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basilio J, Petzelbauer P, Assinger A, Schmid JA (2019) Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front Immunol 10:85. https://doi.org/10.3389/fimmu.2019.00085
Martin A, Komada MR, Sane DC (2003) Abnormal angiogenesis in diabetes mellitus. Med Res Rev 23(2):117–145. https://doi.org/10.1002/med.10024
Mills JL, Sr., Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G, Society for Vascular Surgery Lower Extremity Guidelines C (2014) The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 59(1):220–234e221–222. https://doi.org/10.1016/j.jvs.2013.08.003
Li X (2014) MiR-375, a microRNA related to diabetes. Gene 533(1):1–4. https://doi.org/10.1016/j.gene.2013.09.105
Avnit-Sagi T, Vana T, Walker MD (2012) Transcriptional mechanisms controlling miR-375 gene expression in the pancreas. Exp Diabetes Res 2012:891216. https://doi.org/10.1155/2012/891216
Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J, Zhou T (2010) MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res 20(7):784–793. https://doi.org/10.1038/cr.2010.79
Higuchi C, Nakatsuka A, Eguchi J, Teshigawara S, Kanzaki M, Katayama A, Yamaguchi S, Takahashi N, Murakami K, Ogawa D, Sasaki S, Makino H, Wada J (2015) Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism 64(4):489–497. https://doi.org/10.1016/j.metabol.2014.12.003
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115(5):1111–1119. https://doi.org/10.1172/JCI25102
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106(16):2067–2072. https://doi.org/10.1161/01.cir.0000034509.14906.ae
Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A (2005) Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183(2):259–267. https://doi.org/10.1016/j.atherosclerosis.2005.03.015
Findley CM, Mitchell RG, Duscha BD, Annex BH, Kontos CD (2008) Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J Am Coll Cardiol 52(5):387–393. https://doi.org/10.1016/j.jacc.2008.02.045
Quan A, Pan Y, Singh KK, Polemidiotis J, Teoh H, Leong-Poi H, Verma S (2017) Cardiovascular inflammation is reduced with methotrexate in diabetes. Mol Cell Biochem 432(1–2):159–167. https://doi.org/10.1007/s11010-017-3006-0
Rumore MM, Kim KS (2010) Potential role of salicylates in type 2 diabetes. Ann Pharmacother 44(7–8):1207–1221. https://doi.org/10.1345/aph.1M483
Peiro C, Lorenzo O, Carraro R, Sanchez-Ferrer CF (2017) IL-1beta inhibition in cardiovascular complications associated to diabetes mellitus. Front Pharmacol 8:363. https://doi.org/10.3389/fphar.2017.00363
Wang F, Ge J, Huang S, Zhou C, Sun Z, Song Y, Xu Y, Ji Y (2021) KLF5/LINC00346/miR148a3p axis regulates inflammation and endothelial cell injury in atherosclerosis. Int J Mol Med. https://doi.org/10.3892/ijmm.2021.4985
Miyamoto S, Suzuki T, Muto S, Aizawa K, Kimura A, Mizuno Y, Nagino T, Imai Y, Adachi N, Horikoshi M, Nagai R (2003) Positive and negative regulation of the cardiovascular transcription factor KLF5 by p300 and the oncogenic regulator SET through interaction and acetylation on the DNA-binding domain. Mol Cell Biol 23(23):8528–8541. https://doi.org/10.1128/MCB.23.23.8528-8541.2003
Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I (2005) Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost 3(8):1569–1576. https://doi.org/10.1111/j.1538-7836.2005.01366.x
Ding D, Jiang H, He Y, Li X, Liu X (2021) miR-320-3p regulates the proliferation, migration and apoptosis of hypoxia-induced pulmonary arterial smooth muscle cells via KLF5 and HIF1alpha. Am J Transl Res 13(4):2283–2295
Zhang J, Zheng B, Zhou PP, Zhang RN, He M, Yang Z, Wen JK (2014) Vascular calcification is coupled with phenotypic conversion of vascular smooth muscle cells through Klf5-mediated transactivation of the Runx2 promoter. Biosci Rep 34(6):e00148. https://doi.org/10.1042/BSR20140103
Nan S, Wang Y, Xu C, Wang H (2021) Interfering microRNA-410 attenuates atherosclerosis via the HDAC1/KLF5/IKBalpha/NF-kappaB axis. Mol Ther Nucleic Acids 24:646–657. https://doi.org/10.1016/j.omtn.2021.03.009
Wang J, Zhang L, Wang T, Li C, Jiao L, Zhao Z, Li Y (2021) miRNA-576 alleviates the malignant progression of atherosclerosis through downregulating KLF5. Dis Markers 2021:5450685. https://doi.org/10.1155/2021/5450685
Wang XH, Yan CY, Liu JR (2019) Hyperinsulinemia-induced KLF5 mediates endothelial angiogenic dysfunction in diabetic endothelial cells. J Mol Histol 50(3):239–251. https://doi.org/10.1007/s10735-019-09821-3
Li Y, Li J, Hou Z, Yu Y, Yu B (2016) KLF5 overexpression attenuates cardiomyocyte inflammation induced by oxygen-glucose deprivation/reperfusion through the PPARgamma/PGC-1alpha/TNF-alpha signaling pathway. Biomed Pharmacother 84:940–946. https://doi.org/10.1016/j.biopha.2016.09.100
Ma Y, Wang Q, Liu F, Ma X, Wu L, Guo F, Zhao S, Huang F, Qin G (2018) KLF5 promotes the tumorigenesis and metastatic potential of thyroid cancer cells through the NF-kappaB signaling pathway. Oncol Rep 40(5):2608–2618. https://doi.org/10.3892/or.2018.6687
Chen HL, Chong IW, Lee YC, Tsai JR, Yuan SS, Wang HM, Liu WL, Liu PL (2014) Kruppel-like factor 5 mediates proinflammatory cytokine expression in lipopolysaccharide-induced acute lung injury through upregulation of nuclear factor-kappaB phosphorylation in vitro and in vivo. Mediators Inflamm 2014:281984. https://doi.org/10.1155/2014/281984
Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA (2018) Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical Studies. Stem Cells 36(2):161–171. https://doi.org/10.1002/stem.2751
Khachigian LM (2019) Transcription factors targeted by miRNAs regulating smooth muscle cell growth and intimal thickening after vascular injury. Int J Mol Sci. https://doi.org/10.3390/ijms20215445
Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW (2005) Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res 15(2):92–96. https://doi.org/10.1038/sj.cr.7290271
Kinisu M, Choi YJ, Cattoglio C, Liu K, Roux de Bezieux H, Valbuena R, Pum N, Dudoit S, Huang H, Xuan Z, Kim SY, He L (2021) Klf5 establishes bi-potential cell fate by dual regulation of ICM and TE specification genes. Cell Rep 37(6):109982. https://doi.org/10.1016/j.celrep.2021.109982
Acknowledgements
The authors would like to thank Ana Lay-Hong and Aniket P. Gad for their assistance with immunofluorescence imaging (Harvard Digestive Disease Center, NIH P30DK034854). This work was supported by the National Institutes of Health (Grant Nos. HL115141, HL134849, HL148207, HL148355, HL153356 to M.W.F.), and the American Heart Association (Grant Nos. 18SFRN33900144 and 20SFRN35200163 to M.W.F.; Grant No. 907663 to M.G.M.).
Author information
Authors and Affiliations
Contributions
Conceived the hypothesis: MWF and MGM; performed the experiments: MGM, AJ, GS, HSC, DP-C, RZ, JC; designed or interpreted the results: MGM, AJ, GS, HSC, DP-C, RZ, JC, PPG, MAC, MSS, MPB, and MWF; wrote the manuscript: MGM and MWF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCoy, M.G., Jamaiyar, A., Sausen, G. et al. MicroRNA-375 repression of Kruppel-like factor 5 improves angiogenesis in diabetic critical limb ischemia. Angiogenesis 26, 107–127 (2023). https://doi.org/10.1007/s10456-022-09856-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-022-09856-3