Abstract
Although bronchial angiogenesis has been well documented in allergic asthma, lymphangiogenesis has not been widely studied. Therefore, we evaluated changes in lung lymphatics in a rat model of allergen-induced asthma using house dust mite (Der p 1; 100 μg/challenge). Additionally, properties of isolated lung lymphatic endothelial cells (CD45−, CD141+, LYVE-1+, Prox-1+) were studied in vitro. Three weeks after the onset of intranasal allergen exposure (twice-weekly), an increase in the number of lung lymphatic vessels was measured (34% increase) by lung morphometry. New lymphatic structures were seen predominantly in the peribronchial and periarterial interstitial space but also surrounding large airways. Isolated lymphatic endothelial cells from sensitized lungs showed enhanced proliferation (% Ki67+), chemotaxis, and tube formation (number and length) compared to lymphatic endothelial cells isolated from naive rat lungs. This hyper-proliferative lymphangiogenic phenotype was preserved through multiple cell passages (2–8). Lymphatic endothelial cells isolated from naive and HDM-sensitized rats produced similar in vitro levels of VEGF-C, VEGF-D, and VEGFR3 protein, each recognized as critical lymphangiogenic factors. Inhibition with anti-VEGFR (axitinib, 0.1 μM) blocked proliferation and chemotaxis. Results suggest that in vivo sensitization causes fundamental changes to lymphatic endothelium, which are retained in vitro, and may relate to VEGFR downstream signaling.







Similar content being viewed by others
References
Bailey SR, Boustany S, Burgess JK, Hirst SJ, Sharma HS, Simcock DE, Suravaram PR, Weckmann M (2009) Airway vascular reactivity and vascularisation in human chronic airway disease. Pulm Pharmacol Ther 22:417–425
Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A (2010) Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 65:946–958
Salvato G (2001) Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax 56:902–906
Karmouty-Quintana H, Siddiqui S, Hassan M, Tsuchiya K, Risse PA, Xicota-Vila L, Marti-Solano M, Martin JG (2012) Treatment with a sphingosine-1-phosphate analog inhibits airway remodeling following repeated allergen exposure. Am J Physiol Lung Cell Mol Physiol 302:L736–L745
Van der Velden J, Barker D, Barcham G, Koumoundouros E, Snibson K (2012) Increased vascular density is a persistent feature of airway remodeling in a sheep model of chronic asthma. Exp Lung Res 38:307–315
Chung KF, Rogers DF, Barnes PJ, Evans TW (1990) The role of increased airway microvascular permeability and plasma exudation in asthma. Eur Respir J 3:329–337
Brown R, Mitzner W, Wagner E (1997) Interaction between airway edema and lung inflation on responsiveness of individual airways in vivo. J Appl Physiol 83:366–370
Brown RH, Zerhouni EA, Mitzner W (1995) Airway edema potentiates airway reactivity. J Appl Physiol 79(4):1242–1248
Aebischer D, Iolyeva M, Halin C (2014) The inflammatory response of lymphatic endothelium. Angiogenesis 17:383–393
Ebina M (2008) Remodeling of airway walls in fatal asthmatics decreases lymphatic distribution; beyond thickening of airway smooth muscle layers. Allergol Int 57:165–174
Eifan AO, Orban NT, Jacobson MR, Durham SR (2015) Severe persistent allergic rhinitis: inflammation but no histologic features of structural upper airway remodeling. Am J Respir Crit Care Med 192:1431–1439
Okazaki T, Ni A, Baluk P, Ayeni OA, Kearley J, Coyle AJ, Humbles A, McDonald DM (2009) Capillary defects and exaggerated inflammatory response in the airways of EphA2-deficient mice. Am J Pathol 174:2388–2399
Kretschmer S, Dethlefsen I, Hagner-Benes S, Marsh LM, Garn H, Konig P (2013) Visualization of intrapulmonary lymph vessels in healthy and inflamed murine lung using CD90/Thy-1 as a marker. PLoS ONE 8:e55201
Shin K, Kataru RP, Park HJ, Kwon BI, Kim TW, Hong YK, Lee SH (2015) TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Commun 6:6196
Wagner EM, Jenkins J, Schmieder A, Eldridge L, Zhang Q, Moldobaeva A, Zhang H, Allen JS, Yang X, Mitzner W, Keupp J, Caruthers SD, Wickline SA, Lanza GM (2014) Angiogenesis and airway reactivity in asthmatic brown Norway rats. Angiogenesis 18:1–11
Verloop M (1949) On the arteriae bronchiales and their anastomosing with the arteria pulmonalis in some rodents; a micro-anatomical study. Acta Anat 7:1–32
Butler J (ed) (1992) The bronchial circulation, vol 57. Marcel Dekker Inc, New York
Weibel ER (1960) Early stages in the development of collateral circulation to the lung in the rat. Circ Res 8:353–376
Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC (2010) Indoor air pollution and asthma in children. Proc Am Thorac Soc 7:102–106
Baluk P, McDonald DM (2008) Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci 1131:1–12
Boehme MW, Galle P, Stremmel W (2002) Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals. Immunology 107:340–349
Zhang L, Song K, Zhou L, Xie Z, Zhou P, Zhao Y, Han Y, Xu X, Li P (2015) Heparan sulfate D-glucosaminyl 3-O-sulfotransferase-3B1 (HS3ST3B1) promotes angiogenesis and proliferation by induction of VEGF in acute myeloid leukemia cells. J Cell Biochem 116:1101–1112
Moldobaeva A, Baek A, Eldridge L, Wagner EM (2010) Differential activity of pro-angiogenic CXC chemokines. Microvasc Res 80:18–22
Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15:290–298
Zheng W, Aspelund A, Alitalo K (2014) Lymphangiogenic factors, mechanisms, and applications. J Clin Investig 124:878–887
Randall TD (2010) Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol 107:187–241
Bruyere F, Noel A (2010) Lymphangiogenesis: in vitro and in vivo models. FASEB J 24:8–21
Sweat RS, Sloas DC, Murfee WL (2014) VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model. Microcirc 21:532–540
Kerjaschki D (2014) The lymphatic vasculature revisited. J Clin Investig 124:874–877
Swartz MA, Randolph GJ (2014) Introduction to the special issue on lymphangiogenesis in inflammation. Angiogenesis 17:323–324
Lee E, Pandey NB, Popel AS (2015) Crosstalk between cancer cells and blood endothelial and lymphatic endothelial cells in tumour and organ microenvironment. Expert Rev Mol Med 17:e3
Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM (2005) Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Investig 115:247–257
Yao LC, McDonald DM (2014) Plasticity of airway lymphatics in development and disease. Adv Anat Embryol Cell Biol 214:41–54
Baluk P, Adams A, Phillips K, Feng J, Hong YK, Brown MB, McDonald DM (2014) Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation. Am J Pathol 184:1577–1592
Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM (2009) TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Investig 119:2954–2964
Baluk P, Hogmalm A, Bry M, Alitalo K, Bry K, McDonald DM (2013) Transgenic overexpression of interleukin-1beta induces persistent lymphangiogenesis but not angiogenesis in mouse airways. Am J Pathol 182:1434–1447
Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM (2012) Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 180:2561–2575
Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204:2349–2362
Mori M, Andersson CK, Graham GJ, Lofdahl CG, Erjefalt JS (2013) Increased number and altered phenotype of lymphatic vessels in peripheral lung compartments of patients with COPD. Respir Res 14:65–83
Yamashita M (2015) Lymphangiogenesis and lesion heterogeneity in interstitial lung diseases. Clin Med Insights Circ Respir Pulm Med 9:111–121
Glasgow CG, El-Chemaly S, Moss J (2012) Lymphatics in lymphangioleiomyomatosis and idiopathic pulmonary fibrosis. Eur Respir Rev 21:196–206
Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, Toprak D, Abdelnour E, D’Agostino E, Goldberg HJ, Perrella MA, Forteza RM, Rosas IO, Visner G, El-Chemaly S (2015) Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Investig 125:4255–4268
Leak LV, Jamuar MP (1983) Ultrastructure of pulmonary lymphatic vessels. Am Rev Respir Dis 128:S59–S65
Ohtani O, Ohtani Y (2008) Organization and developmental aspects of lymphatic vessels. Arch Histol Cytol 71:1–22
Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K (2002) Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21:4593–4599
Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G (2002) Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225:351–357
Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G (2008) Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22:3282–3291
Sweat RS, Stapor PC, Murfee WL (2012) Relationships between lymphangiogenesis and angiogenesis during inflammation in rat mesentery microvascular networks. Lymphat Res Biol 10:198–207
Rafii S, Butler JM, Ding BS (2016) Angiocrine functions of organ-specific endothelial cells. Nature 529:316–325
Podgrabinska S, Skobe M (2014) Role of lymphatic vasculature in regional and distant metastases. Microvasc Res 95:46–52
Shinoda K, Hirahara K, Iinuma T, Ichikawa T, Suzuki AS, Sugaya K, Tumes DJ, Yamamoto H, Hara T, Tani-Ichi S, Ikuta K, Okamoto Y, Nakayama T (2016) Thy1+IL-7+lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc Natl Acad Sci USA 113:E2842–E2851
Acknowledgements
Funding was provided by National Heart, Lung, and Blood Institute (Grant Nos. HL10342 and HL113392).
Authors’ contributions
A.M. and E.M.W. were involved in conception and design. A.M., J.J., and Q.Z. carried out experimental work. A.M. and E.M.W. performed analysis and interpretation. A.M. and E.M.W. finalized manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
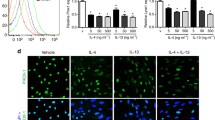
Lymphatic endothelial cells in culture. A. Bright-field exposure showing cobblestone morphology of confluent monolayer; B. anti-LYVE-1+ staining (green), nuclei were counterstained with DAPI (blue) at enhanced magnification; and C. anti-Prox-1+ nuclei. Bar = 50 μm (TIFF 1536 kb)
Rights and permissions
About this article
Cite this article
Moldobaeva, A., Jenkins, J., Zhong, Q. et al. Lymphangiogenesis in rat asthma model. Angiogenesis 20, 73–84 (2017). https://doi.org/10.1007/s10456-016-9529-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-016-9529-2