Abstract
Considerable efforts have been made to amplify angiogenesis under conditions of hypoxia and ischemia by vascular endothelial growth factor (VEGF) delivery, so far with limited success. Ischemic vascular diseases are often associated with hypercholesterolemia. To elucidate whether the exposure to blood lipids influences VEGF responses of microvessels, we characterized effects of low density lipoprotein (LDL) exposure on the proliferation, migration and tube formation of human umbilical vein endothelial cells. By examining the expression, phosphorylation and downstream signals of VEGF’s receptor VEGFR2, we characterized mechanisms controlling angiogenic responses following LDL exposure. LDL attenuated endothelial proliferation, migration and tube formation in a dose-dependent way. Reduced abundance of VEGFR2 and VEGFR1 were noticed in LDL-exposed endothelial cells. In subcellular localization studies that we combined with pharmacological experiments, we showed that the loss of VEGFR2 resulted from its internalization and degradation, the latter of which required syntaxin-16-dependent endosome-trans-Golgi network trafficking. As a consequence, VEGFR2 phosphorylation and downstream signals -specifically Akt and ERK1/2 phosphorylation- were attenuated in response to VEGF treatment. VEGF only partly reversed the effects of LDL on angiogenesis under conditions of normoxia and hypoxia. Our results suggest that angiogenic responses to VEGF are compromised in hypercholesterolemia as a consequence of endosomal VEGFR2 degradation.






Similar content being viewed by others
References
Mäkinen K, Manninen H, Hedman M, Matsi P, Mussalo H, Alhava E, Ylä-Herttuala S (2002) Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther 6:127–133
Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER (2003) The VIVA trial: vascular endothelial growth factor in Ischemia for vascular angiogenesis. Circulation 107:1359–1365
Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A, Vanninen E, Mussalo H, Kauppila E, Simula S, Narvanen O, Rantala A, Peuhkurinen K, Nieminen MS, Laakso M, Yla-Herttuala S (2003) Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of post angioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio angiogenesis trial. Circulation 107:2677–2683
Kukuła K, Chojnowska L, Dąbrowski M, Witkowski A, Chmielak Z, Skwarek M, Kądziela J, Teresińska A, Małecki M, Janik P, Lewandowski Z, Kłopotowski M, Wnuk J, Rużyłło W (2011) Intramyocardial plasmid-encoding human vascular endothelial growth factor A165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (VIF-CAD). Am Heart J 161:581–589
Rajagopalan S, Mohler ER III, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH (2003) Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 108:1933–1938
Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, Bøtker HE, Dudek D, Drvota V, Hesse B, Thuesen L, Blomberg P, Gyöngyösi M, Sylvén C, Euroinject One Group (2005) Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol 45:982–988
Simons M, Ware JA (2003) Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov 2:863–871
Zhang ZG, Chopp M (2009) Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 8:491–500
Hermann DM, Zechariah A (2009) Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab 29:1620–1643
Hermann DM, Chopp M (2012) Promoting brain remodeling and plasticity for stroke recovery: therapeutic potential, caveats and consequences for clinical translation. Lancet Neurol 11:369–380
Iso H, Jacobs DR Jr, Wentworth D, Neaton JD, Cohen JD (1989) Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 320:904–910
Horenstein RB, Smith DE, Mosca L (2002) Cholesterol predicts stroke mortality in the Women’s Pooling Project. Stroke 33:1863–1868
Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T (2000) Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation 102:III370–III376
Matter CM, Ma L, von Lukowicz T, Meier P, Lohmann C, Zhang D, Kilic U, Hofmann E, Ha SW, Hersberger M, Hermann DM, Lüscher TF (2006) Increased balloon-induced inflammation, proliferation, and neointima formation in apolipoprotein E (ApoE) knockout mice. Stroke 37:2625–2632
Osto E, Matter CM, Kouroedov A, Malinski T, Bachschmid M, Camici GG, Kilic U, Stallmach T, Boren J, Iliceto S, Lüscher TF, Cosentino F (2008) c-Jun N-terminal kinase 2 deficiency protects against hypercholesterolemia-induced endothelial dysfunction and oxidative stress. Circulation 118:2073–2080
ElAli A, Doeppner TR, Zechariah A, Hermann DM (2011) Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 42:3238–3244
Van Belle E, Rivard A, Chen D, Silver M, Bunting S, Ferrara N, Symes JF, Bauters C, Isner JM (1997) Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation 96:2667–2674
Jang JJ, Ho HK, Kwan HH, Fajardo LF, Cooke JP (2000) Angiogenesis is impaired by hypercholesterolemia: role of asymmetric dimethylarginine. Circulation 102:1414–1419
Tirziu D, Moodie KL, Zhuang ZW, Singer K, Helisch A, Dunn JF, Li W, Singh J, Simons M (2005) Delayed arteriogenesis in hypercholesterolemic mice. Circulation 112:2501–2509
Chen CH, Jiang W, Via DP, Luo S, Li TR, Lee YT, Henry PD (2000) Oxidized low-density lipoproteins inhibit endothelial cell proliferation by suppressing basic fibroblast growth factor expression. Circulation 101:171–177
Chang PY, Luo S, Jiang T, Lee YT, Lu SC, Henry PD, Chen CH (2001) Oxidized low-density lipoprotein downregulates endothelial basic fibroblast growth factor through a pertussis toxin-sensitive G-protein pathway: mediator role of platelet-activating factor-like phospholipids. Circulation 104:588–593
Chavakis E, Dernbach E, Hermann C, Mondorf UF, Zeiher AM, Dimmeler S (2001) Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation 103:2102–2107
Sata M, Walsh K (1998) LDL activates Fas-medited endothelial cell apoptosis. J Clin Invest 102:1682–1689
Salvayre R, Auge N, Benoist H, Negre-Salvayre A (2002) Oxidized low-density lipoprotein-induced apoptosis. Biochim Biophys Acta 1585:213–221
Itabe H (2003) Oxidized low-density lipoproteins: what is understood and what remains to be clarified. Biol Pharm Bull 26:1–9
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343
Zeng H, Dvorak HF, Mukhopadhyay D (2001) Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) receptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem 276:26969–26979
Kitamura T, Asai N, Enomoto A, Maeda K, Kato T, Ishida M, Jiang P, Watanabe T, Usukura J, Kondo T, Constantini F, Murohara T, Takahashi M (2008) Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol 10:329–337
Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ (2006) ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 9:33–44
Bruns AF, Herbert SP, Odell AF, Jopling HM, Hooper NM, Zachary IC, Walker JH, Ponnambalam S (2010) Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic 11:161–174
Mukherjee S, Tessema M, Wandinger-Ness A (2006) Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res 98:743–756
Laufman O, Hong W, Lev S (2011) The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J Cell Biol 194:459–472
Dong Y, Wu Y, Wu M, Wang S, Zhang J, Xie Z, Xu J, Song P, Wilson K, Zhao Z, Lyons T, Zou MH (2009) Activation of protease calpain by oxidized and glycated LDL increases the degradation of endothelial nitric oxide synthase. J Cell Mol Med 13:2899–2910
Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J (2012) The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler Thromb Vasc Biol 32:1167–1177
Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N (2011) PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol Cell Biol 31:2010–2025
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Salomonsson L, Svensson L, Pettersson S, Wiklund O, Ohlsson BG (2003) Oxidised LDL decreases VEGFR-1 expression in human monocyte-derived macrophages. Atherosclerosis 169:259–267
Shibuya M (2006) Vascular endothelial growth factor (VEGF)-receptor 2: its biological functions, major signaling pathway, and specific ligand VEGF-E. Endothelium 13:63–69
Roskoski R Jr (2008) VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun 375:287–291
Usui R, Shibuya M, Ishibashi S, Maru Y (2007) Ligand-independent activation of vascular endothelial growth factor receptor 1 by low-density lipoprotein. EMBO Rep 8:1155–1161
Manickam V, Tiwari A, Jung JJ, Bhattacharya R, Goel A, Mukhopadhyay D, Choudhury A (2011) Regulation of vascular endothelial growth factor receptor 2 trafficking and angiogenesis by Golgi localized t-SNARE syntaxin 6. Blood 117:1425–1435
Avraham-Davidi I, Ely Y, Pham VN, Castranova D, Grunspan M, Malkinson G, Gibbs-Bar L, Mayseless O, Allmog G, Lo B, Warren CM, Chen TT, Ungos J, Kidd K, Shaw K, Rogachev I, Wan W, Murphy PM, Farber SA, Carmel L, Shelness GS, Iruela-Arispe ML, Weinstein BM, Yaniv K (2012) ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat Med 18:967–973
Acknowledgments
We gratefully acknowledge Prof. E. Metzen, Department of Physiology, University of Duisburg-Essen, for providing laboratory space and advise for cell culture works. This work was supported by the German Research Foundation (HE3173/2-1 and HE3173/3-1; to D.M.H.), Dr.Werner-Jackstädt Foundation (to F.J.) and Heinz-Nixdorf Foundation (to D.M.H.).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2013_9340_MOESM1_ESM.tif
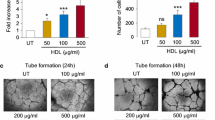
Figure 1. LDL decreases VEGFR2 protein abundance, at the same time increasing VEGFR2 mRNA level. (A) Western blots for VEGFR2, for phosphorylated (i.e., activated) Akt and total Akt, for phosphorylated (i.e., activated) ERK1/2 and total ERK1/2, and for the house keeping protein α-tubulin of whole cell lysates of endothelial cells exposed to different LDL concentrations (25-100 μg/ml) for various durations (6 h-48 h). Note the reduced VEGFR2 abundance after LDL exposure for 24 and 48 h. (B) Real-time PCR for vegfr2 mRNA normalized with gapdh mRNA of endothelial cells exposed to different LDL concentrations (25-100 μg/ml) for various durations (6 h-48 h). Note that vegfr2 mRNA is not reduced, but increased following LDL exposure. *p < 0.05/**p < 0.01/***p < 0.001 versus control (n ≥ 3 independent experiments). (TIFF 294 kb)
10456_2013_9340_MOESM2_ESM.tif
Figure 2. LDL reduces VEGFR1 and VEGFR2 protein abundance. Western blots and quantification for (A) VEGFR1 and the house keeping protein α-tubulin and (B) VEGFR2 and the house keeping protein β-actin of whole whole cell lysates of endothelial cells exposed to different LDL concentrations (25-100 μg/ml in A, 100-2,500 µg/ml in B) for 72 h (in A) or 24 h (in B). Note the reduced VEGFR1 and VEGFR2 abundance that in case of VEGFR2 was now evaluated over a wide concentration range, thus mimicking conditions of hypercholesterolemia. *p < 0.05/**p < 0.01/***p < 0.001 versus control (n ≥ 3 independent experiments). (TIFF 108 kb)
10456_2013_9340_MOESM3_ESM.tif
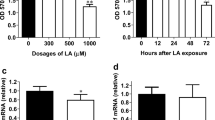
Figure 3. VEGFR2 is not degraded by calpains, matrix metalloproteinases, the ubiquitin–proteasome system and caspase-3. (A) Calpain and (B) MMP3 activity determined by calpain or MMP3 activity kits. Endothelial cells were harvested at indicated time points after LDL exposure (100 µg/ml) and whole cell lysates were examined. No significant changes in calpain or MMP3 activity are detectable. (C) MMP2 and MMP9 activity evaluated by gelatin zymography of whole cell lysates of endothelial cells exposed to LDL (100 µg/ml) for 12 to 72 h. Note the presence of MMP2 (72 kDa, arrowhead) but absence of MMP9 (92 kDa, arrow) that do not change in response to LDL exposure. (D) Western blots for VEGFR2, caspase-3, cleaved (i.e., activated caspase-3) and β-actin (which was used as house keeping protein) of whole cell lysates of cells exposed to LDL (100 µg/ml) for 12 to 72 h. Note the absence of caspase-3 cleavage (i.e., activation) in response to LDL exposure. (E) Immunoprecipitation assay for VEGFR2 that was detected with a ubiquitin antibody of whole cell lysates of cells exposed to LDL (100 µg/ml) for 1 h. Note the absence of ubiquitinated VEGFR2 following LDL exposure. (F) Western blots for VEGFR2 and the house keeping protein β-actin of whole cell lysates of endothelial cells that were incubated with different inhibitors of enzymatic degradation systems 1 h prior to LDL exposure (100 µg/ml). Note that inhibition of calpains (calpeptin; 1 µM), MMPs (TIMP-1; 50 ng/ml), the ubiquitin–proteasome system (MG132; 70 nM) and caspase-3 (z-DEVD-FMK; 2 µM) does not abrogate the LDL-induced VEGFR2 degradation. Throughout the studies, no significant differences were noticed between groups (n = 3 independent experiments). (TIFF 301 kb)
10456_2013_9340_MOESM4_ESM.tif
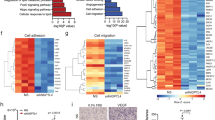
Figure 4. VEGFR2 is not a target gene of HIF-1α or HIF-2α. (A, B) Western blots for VEGFR2, HIF-1α, HIF-2α and the house keeping protein α-tubulin of whole cell lysates of endothelial cells exposed to either 21 % oxygen (normoxia) or 1 % oxygen (hypoxia) for various durations (2-24 h) (in A) or 24 h (in B). In (B), HIF-1α and HIF-2α were knocked down with pLKO.1-shRNA-HIF-1α or pLKO.1-shRNA-HIF-2α. Note that the knockdown of HIF-1α and HIF-2α, which are both induced upon hypoxia, does not alter VEGFR2 abundance. No significant differences in VEGFR2 abundance were noticed between groups. (TIFF 132 kb)
Rights and permissions
About this article
Cite this article
Jin, F., Hagemann, N., Brockmeier, U. et al. LDL attenuates VEGF-induced angiogenesis via mechanisms involving VEGFR2 internalization and degradation following endosome-trans-Golgi network trafficking. Angiogenesis 16, 625–637 (2013). https://doi.org/10.1007/s10456-013-9340-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9340-2