Abstract
The fibrinous exudate of a wound or tumor stroma facilitates angiogenesis. We studied the involvement of RGD-binding integrins during tube formation in human plasma-derived fibrin clots and human purified fibrin matrices. Capillary-like tube formation by human microvascular endothelial cells in a 3D plasma-derived fibrinous matrix was induced by FGF-2 and TNF-α and depended largely on cell-bound u-PA and plasmin activities. While tube formation was minimally affected by the addition of either the αvβ3-integrin inhibiting mAb LM609 or the α5-integrin inhibiting mAb IIA1, the general RGD-antagonist echistatin completely inhibited this process. Remarkably, when αvβ3- and α5β1-integrins were inhibited simultaneously, tube formation was reduced by 78%. It was accompanied by a 44% reduction of u-PA antigen accumulation and 41% less production of fibrin degradation products. αvβ5-integrin-blocking antibodies further enhanced the inhibition by mAb LM609 and mAb IIA1 to 94%, but had no effect by themselves. αv-specific cRGD only inhibited angiogenesis when α5β1-integrin was simultaneously blocked. Endostatin mimicked the effect of α5β1-integrin and inhibited tube formation only in the presence of LM609 or cRGD (73 and 80%, respectively). Comparable results were obtained when purified fibrin matrices were used instead of the plasma-derived fibrinous matrices. These data show that blocking of tube formation in a fibrinous exudate requires the simultaneous inhibition of αvβ3- and α5β1-integrins. This may bear impact on attempts to influence angiogenesis in a fibrinous environment.
Similar content being viewed by others
Introduction
Angiogenesis, the outgrowth of new microvascular structures from the preexisting vasculature, is pivotal for embryonic development [1]. Once the organism has become adult, angiogenesis is limited to the endometrium and ovary. However, as part of normal or deranged tissue-repair processes, it is induced after wounding and in a number of diseases, such as cancer, diabetic retinopathy, and rheumatoid arthritis [1, 2]. Repair-associated angiogenesis is normally accompanied by fibrin deposition and accumulation of cytokine-producing leukocytes [3–6]. The leakiness of tumor vessels and the presence of tissue factor in the interstitial tissue favor the deposition of a fibrinous matrix as part of the tumor stroma. Such a fibrinous matrix provides excellent scaffolding for the invasion of new vascular structures [4, 7, 8]. Thus, the temporary fibrinous scaffolding not only plays an important role in wound closure, but also supports cell invasion and angiogenesis in tumor stroma and in inflamed tissues.
The induction and activity of cell-bound proteolytic activities and matrix-binding receptors largely regulate the migration and invasion of endothelial cells during angiogenesis, in addition to structural epitopes in the extracellular or fibrinous matrix itself [9]. Both the cell-bound urokinase and plasmin activities and matrix metalloproteinases (MMPs), in particular membrane-type MMPs (MT-MMPs), play a pivotal role in migration and invasion of endothelial cells during the formation of tubular structures in a fibrin matrix [9–14].
Besides proteolysis, the formation of new adhesion sites between the invading cells and the matrix is required. Among the various types of adhesion molecules, the integrins particularly play an important role in tissue repair and tumor-induced angiogenesis [15–18]. Integrins are transmembrane glycoproteins consisting of noncovalently linked α- and β-subunits, which can form various heterodimers [18, 19]. Presently, nine integrins have been recognized on endothelial cells [19]. The RGD-recognizing integrins αvβ3-, αvβ5-, and α5β1 facilitate cell binding to fibrin [20] and are expressed on the endothelial cells of newly formed vessels in a fibrinous matrix of wounds and tumors [21–23]. Quantitative alterations in αvβ3- and α5β1-integrin expression in particular, modulate the adhesive and migratory properties of endothelial cells during angiogenesis in wound repair and tumor growth [24, 25]. This can be induced by provisional matrix molecules in the wound clot, such as fibrin, and local conditions, such as hypoxia [26, 27]. On the basis of the inhibitory activities of αv- and αvβ3-integrin antagonists, such as cRGD peptides and antibody LM609, on angiogenesis in several pathological conditions in animals it was postulated that such antagonist may be good candidates for inhibiting tumor angiogenesis [21]. However, this concept was challenged by the finding of Reynolds et al. [28, 29] that mice lacking β3-integrins or both β3- and β5-integrins support tumor angiogenesis and tumor growth. This data indicates that the role of αv-integrins in angiogenesis is more complex than originally thought [30–32]. In addition to αvβ3 inhibition, blocking of α5β1 can contribute to the regulation of angiogenesis [23]. The α5β1-integrin and other integrins have been shown to be targets for endostatin, the C-terminal fragment of collagen XVIII [33, 34] which can strongly suppress tumor-induced angiogenesis in animals [35–37].
The formation of capillary-like structures in a fibrinous exudate can be mimicked by human microvascular endothelial cells (hMVECs) in a 3D-fibrin matrix [11]. With this model we reported previously that inhibition of αvβ3-integrin activity by LM609 did not reduce the formation of capillary-like structures by hMVECs in a fibrin matrix [27]. Subsequently we observed that inhibition of RGD-binding integrins by echistatin prevented tube formation. Because both α5β1- and αv-integrins are involved in the binding of endothelial cells to a fibrinous matrix and may influence their activities mutually, we further evaluated how various RGD-binding integrins cooperate in the formation of capillary-like structures. This was studied both in a fibrin matrix and in 3D-matrices consisting of a plasma clot to mimic the fibrinous exudate more closely. The involvement of RGD-binding integrins during neovascularization could also be demonstrated by tube formation in the murine fibrinous exudate.
Materials and methods
Materials
Cell culture reagents were purchased as previously described [38]. Human plasma and serum were obtained from a local blood bank, and plasma clot matrices were prepared from freshly obtained blood from 10 to 20 healthy donors. FGF-2 was purchased from Pepro Tech EC (London, UK) and human recombinant TNF-α from Sigma–Aldrich Chemie (Steinheim, Germany). Factor XIII was generously provided by Dr. H. Metzner and Dr. G. Seeman (Aventis Behring GmbH, Marburg, Germany). Thrombin was purchased from Leo Pharmaceutic Products (Weesp, The Netherlands). The active αvβ3-blocking mAb LM609 and the αvβ5-blocking mAb P1F6 were purchased from Chemicon (Temecula, CA). The α5-integrin blocking mAb IIA1 was obtained from Pharmingen (Hamburg, Germany). The α1-integrin blocking mAb 5E8D9 was obtained from Upstate (Milton Keynes, UK). Endostatin was produced as described by Yamaguchi et al. [36]. The biotinylated horse anti-mouse IgG was obtained from Vector Laboratories (Burlingame, CA) and the biotinylated donkey anti-rabbit was purchased from Amersham Pharmacia Biotech UK (Buckinghamshire, England). Rabbit anti-α5 and -β5 integrins, both against the cytoplasmatic domain of the integrin, were kindly provided by Dr. Guido Tarone (University of Turin, Italy). The blocking anti-β1 mAb P4C10 was obtained from Millipore (Billerica, MA). The inhibitory anti-uPAR mAb H-2 was kindly donated by Dr. U. Weidle (Boehringer Mannheim, Penzberg, Germany). The human CD31 antigen, a monoclonal antibody, was from Novocastra Laboratories, Newcastle, UK. Pepsin was purchased from Sigma–Aldrich Chemie (Steinheim, Germany). Biotinylated thyramides were obtained from the Department of Endocrinology from the Leiden University Medical Center (The Netherlands). The horseradish peroxidase (HRP) Avidin Biotinylated Complex was purchased from DAKO (Glostrup, Denmark) and Novared from Vector Laboratories (Burlingame, CA). Time specimens of recanalized mural thrombi were kindly provided by Dr. H·W. Niessen (VU University Medical Center Amsterdam, Department of Pathology, Amsterdam, The Netherlands). cRGD, an αv-inhibiting peptide and GRGDSP, an RGD-inhibiting peptide were obtained from Bachem (St. Helens, UK).
Cell culture
Human foreskin microvascular cells (hMVECs) were isolated, cultured and characterized as previously described [39, 40].
Immunohistochemical analysis
Tissue sections of a recanalized human mural thrombus were dewaxed by immersion in xylene and rehydrated in decreasing concentrations of ethanol. For HPS staining, the sections were counterstained with Mayer’s hematoxylin, phloxin and saffron. After dehydration in a reversed ethanol–xylene series, the sections were prepared for microscopy.
Inhibition for endogenous peroxidase was accomplished by immersion in 1% hydrogen peroxidase in absolute methanol for 20 min at room temperature. The sections were washed in deionized water and equilibrated in PBS. Antigen retrieval for sections treated with anti-CD31 was successful when the sections were incubated in 0.1 mol/l sodium-citrate in a microwave at 700 W until boiling point was reached, followed by a period of 10 min at 180 W. In the case of LM609 and the rabbit anti-human antibody against the α5-integrin, appropriate antigen retrieval was reached when the sections were treated with 2 mg/ml pepsin for 75 min at room temperature. For sections treated with the rabbit anti-human antibody against the β5-integrin, appropriate antigen retrieval was reached after a 10-min incubation at room temperature with proteinase K. Subsequently, all the sections were blocked by 5% BSA in PBS to prevent nonspecific binding. Incubation with the anti-CD31 (1:100), LM609 (10 μg/ml), anti-α5 (whole rabbit serum diluted 1:400), and anti-β5 (whole rabbit serum diluted 1:50) was done overnight at 4°C. After washes with PBS, the sections were exposed to the second antibody, the biotinylated horse anti-mouse IgG (1:400) or the donkey anti-rabbit (1:200) diluted in 1% BSA/PBS, for 1 h at room temperature. Afterwards, the sections were washed with PBS and incubated for 30 min at room temperature with the HRP Avidin Biotinylated Complex (ABComplex). In order to amplify the signal of the sections stained with the anti-β5-integrin, biotinylated thyramides were added for 10 min at room temperature, followed again by the HRP ABComplex for 30 min at room temperature. The sections were washed with PBS and stained with Novared for a period of 5–10 min. Finally, the sections were washed in aquadest, counterstained with hematoxylin, washed in running water and dehydrated in a reversed ethanol–xylene series, and prepared for microscopy.
Preparation of the three-dimensional plasma and fibrin clots
Coagulation of plasma clots was performed as described by Engelse et al. [41]. Briefly, 2.5 U/ml Factor XIII and 1 U/ml thrombin were added to human plasma, and 300 μl aliquots of this mixture was added to the wells of a 48-well plate. After clotting at room temperature for at least 30 min, the matrices were equilibrated at 37°C, 5% CO2/95% air atmosphere with serum-containing M199 to inactivate the thrombin. During the next 24 h, the matrices were washed three times with M199 to eliminate residual citrate. Fibrin matrices were prepared as previously described [11].
In vitro tube formation assay
The formation of capillary-like tubes in plasma-derived fibrinous matrices was evaluated essentially as previously described in fibrin matrices [11]. Confluent hMVECs were seeded on top of the matrices. After seeding 24 h, the cells were stimulated with serum-containing M199 with or without (control) the presence of FGF-2 and TNF-α and the reagents to be tested. Every second day, the medium was removed and replaced by fresh stimulation medium and after 7 days, the formation of tubular structures was analyzed by phase contrast microscopy as previously described [11].
ELISAs
u-PA antigen determinations as well as fibrin degradation products (FDP) determinations were performed by the commercially available immunoassay kits: u-PA EIA HS (Taurus, Leiden, The Netherlands) and Fibrinostika® FDP ELISA (Organon-Technika, Turnhout, Belgium). The monoclonal antibodies that are used in this uPA ELISA were made in our department and recognize all forms of uPA: latent (single-chain) uPA, active (two-chain) uPA, and u-PA complexed with PAI-1 in a similar manner [11, 42].
Statistical analysis
Experiments were performed in duplicate and the results were expressed as mean ± SEM in relation to the FGF-2/TNF-α condition. For statistical analysis, we used one-way ANOVA followed by the Dunnett’s test as post-test. The Dunnett’s test is a modified t-test that takes into account multiple comparisons with a control condition. Statistical significance was accepted at P < 0.05.
Results
Endothelial cells in a fibrinous matrix express αvβ3-, αvβ5- and α5β1-integrins
Endothelial cells produce several integrins, three of which have been reported to interact with fibrin: αvβ5-, αvβ3-, and α5β1-integrins. To verify the presence of these integrins in new vascular structures formed in a fibrinous environment, immunohistochemical analysis of these integrins was performed in tissue sections of a recanalized human mural thrombus. As is shown in Fig. 1, αvβ3-integrin, β5-containing integrins reflecting αvβ5-integrin, and α5-containing integrins, representing α5β1 integrin, were present in many vascular structures of these thrombi.
Localization of RGD-binding integrins in endothelial cells in vivo. Immunohistochemistry was performed on paraffin sections of a recanalized mural thrombus formed in a human coronary artery as described in Materials and Methods. a Phloxin staining; b Immunostaining of endothelial cells by CD31; c Immunostaining of αvβ3-integrin by mAb LM609; d Immunostaining of αvβ5-integrin by rabbit anti-human β5-integrin antibody; e Immunostaining of α5β1-integrin by rabbit anti-human α5-integrin antibody
Involvement of RGD-binding integrins in the formation of tubular structures in a 3D plasma-derived fibrinous matrix
To investigate the involvement of integrins in tube formation in a human fibrinous exudate, we used an in vitro matrix consisting of clotted plasma to study the formation of capillary-like tubes by human microvascular endothelial cells (hMVECs). For that, hMVECs were cultured on top of the plasma-derived fibrinous matrix, and capillary-like structures were formed after 4–6 days of culture in the continuous presence of both FGF-2 (10 ng/ml) and TNF-α (10 ng/ml), whereas addition of either FGF-2 or the cytokine TNF-α hardly induced the formation of tubular structures comparable to previous observations on hMVEC-mediated tube formation in plasma-derived fibrinous matrices [41] and fibrin matrices made using purified fibrinogen [11].
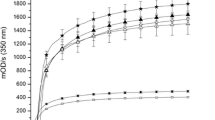
When echistatin (1 μg/ml), a general RGD inhibitor, was added simultaneously with FGF-2/TNF-α to hMVECs grown on a human plasma-derived fibrinous matrix, hardly any capillary-like structures were formed (18.6 ± 4.7% of the amount formed by FGF-2/TNF-α-stimulated cells, n = 3, P < 0.001). The nature of the RGD-binding integrins was further investigated using blocking mAbs against α5β1-, αvβ3-, and αvβ5-integrins. Addition of either the αvβ3-integrin blocking mAb LM609, the αvβ5-integrin antagonist mAb P1F6, or the α5-integrin inhibiting mAb IIA1 did not or only marginally affect the formation of tubular structures in a plasma clot (Fig. 2a). Their effectiveness was verified by their ability to inhibit cell binding to fibronectin by 61 ± 6% (mAb IIA1) or vitronectin-coated dishes by 55 ± 7% (mAb LM609) and 40 ± 14% (mAb P1F6). Interestingly, when mAb IIA1 was added together with either LM609 or P1F6, the formation of capillary-like structures was significantly decreased. The combination of anti-αvβ3 (LM609) and anti-α5 (IIA1) integrins was most effective. Addition of anti-αvβ5 P1F6 to the combination of mAb LM609 and mAb IIA1 resulted in a further reduction of the formation of capillary-like structures (Fig. 2a). Another mAb, 5E8D9, that blocks the α1-integrin, did not affect tube formation neither by itself nor in combination with mAb LM609 or mAb IIA1 (86 ± 2 and 82 ± 1 of control, respectively). The β1-integrin blocking mAb P4C10, which inhibits α5β1- and α3β1-integrins, had no effect on tube formation when added as a single antibody (data not shown).
Blocking of αvβ3- and α5β1-integrins results in a combined effect on the inhibition of capillary-like tube formation in plasma-derived fibrinous matrices. HMVECs cultured on a three-dimensional fibrinous plasma clot were not stimulated (control) or stimulated with FGF-2/TNF-α in the absence or presence of the αvβ3-blocking mAb LM609 (10 μg/ml), the αvβ5-blocking mAb P1F6 (10 μg/ml), the α5-blocking mAb IIA1 (2 μg/ml) or the combinations of these mAbs. After 6 days of culturing, the mean tube length of duplicate wells was quantified (mm/cm2) as described (a). The data represent mean % ± SEM of FGF-2/TNF-α stimulated tube formation of 3–7 independent experiments. The mean tube length of FGF-2/TNF-α in the plasma-derived fibrinous matrix was 70 ± 20 mm/cm2. b After 6 days of culturing, non-phase photomicrographs were taken of hMVECs stimulated with FGF-2/TNF-α in the absence or presence of LM609, IIA1 or the combination of these mAbs in the concentrations described above. Bar represents 300 μm. * P < 0.01 as compared to 100% (=FGF-2/TNF-α stimulated condition)
The presence of either mAb LM609 or mAb IIA1 during the tube formation assay did not affect cell attachment, once the cells had been seeded on top of a plasma-derived fibrinous matrix (compare the intact monolayer of cells in Fig. 2b). No apoptotic cells were observed in the presence of integrin inhibitors as determined by the absence of fragmentation of nuclei, after DAPI staining and evaluation by digital imaging microscopy (data not shown).
Simultaneous addition of mAb LM609 and mAb IIA1 affects u-PA and FDP production
The reduced ingrowth of capillary-like structures after exposure to mAb LM609 and mAb IIA1 was accompanied by a reduced accumulation of u-PA in the conditioned medium of the tube-forming cells (Table 1). Furthermore, the reduced formation of tubular structures was paralleled by a decreased generation of fibrin degradation products (Table 1). The individual antibodies hardly affected these parameters. This suggests that the simultaneous interference with αvβ3- and α5β1-integrins results in a diminished u-PA accumulation in the supernatants of the FGF-2/TNF-α-treated cells and a diminished generation of FDP products. Experiments using the uPA receptor (uPAR) blocking antibody H-2 indicated that the differences in uPA were not due to decreased uPAR availability on the endothelial cells. The mAb H-2 displaces uPA from the uPAR and thereby prevents the uptake and degradation of uPA by the endothelial cells. In the presence of mAb H-2, the accumulation of uPA in the conditioned medium increased by 160–180% in all conditions as compared with each counterpart without H-2.
Endostatin acts in concert with mAb LM609 and cRGD in inhibiting capillary-like tube formation
Endostatin, at a concentration of 10 μg/ml, affected the FGF-2/TNF-α-induced formation of tubular structures by hMVECs in a plasma-derived fibrinous matrix minimally (Fig. 3). When endostatin was given simultaneously with mAb LM609, but not with mAb IIA1, the formation of tubular structures was largely inhibited (Fig. 3). Apparently, endostatin was able to mimic the effect of a α5-integrin blocking e.g., mAb IIA1 (compare Fig. 3 and 2a).
Combined effect of endostatin, LM609, and/or cRGD on capillary-like tube formation in fibrinous plasma clots. HMVECs cultured on a three-dimensional plasma clot were not stimulated (control) or stimulated with FGF-2/TNF-α in the presence of either endostatin (10 μg/ml), the αvβ3-blocking mAb LM609 (10 μg/ml), the α5-blocking mAb IIA1 (2 μg/ml) or cRGD (50 μg/ml), a combination of endostatin and LM609, endostatin and IIA1, cRGD and endostatin, or IIA1 and cRGD. After 6 days of culturing, the mean tube length was quantified (mm/cm2) as described. The data represent mean % ± SEM of FGF-2/TNF-α stimulated tube formation of 3–5 independent experiments. The mean tube length of FGF-2/TNF-α was 98 ± 15 mm/cm2. * P < 0.05, ** P < 0.01 as compared to 100% (=FGF-2/TNF-α stimulated condition)
The interaction of α5β1- and αvβ3-integrins was further underscored using cyclic RGD peptides with specificity for αv-integrins [43]. While the formation of capillary-like structures in the plasma clot was minimally affected by the addition of cRGD or endostatin alone, tube formation was largely inhibited when cRGD and endostatin were added simultaneously (Fig. 3). A similar inhibition was achieved by simultaneous addition of cRGD and mAb IIA1 (Fig. 3). These results indicate that cRGD and endostatin inhibited the formation of tubes to the same extent as mAb LM609 and endostatin.
Tube formation in a fibrinous exudate requires interaction between fibrin and RGD-binding integrins
The plasma clot consists of various matrix proteins that contain RGD-sequences, in particular fibrin, fibronectin and vitronectin. While the RGD-sequences in vitronectin of human and mice are comparably organized, the RGD-sequences in the Aα-chain of fibrinogen are markedly different. Therefore, we investigated whether the effects of integrin inhibitors were comparable, when hMVECs formed tubular structures in a fibrin matrix prepared from purified fibrinogen. As is shown in Fig. 4, addition of integrin inhibitors, in particular against α5β1- and αvβ3-integrins, largely reduced tube formation in a fibrin matrix. These data suggest that fibrin largely determines integrin requirement for tube formation in a plasma-derived fibrinous matrix.
Blocking of αvβ3- and α5β1-integrins results in a combined effect on the inhibition of capillary-like tube formation in purified fibrin matrices. HMVECs cultured on a three-dimensional fibrin matrix and were not stimulated (control) or stimulated with FGF-2/TNF-α in the absence or presence of the αvβ3-blocking mAb LM609 (10 μg/ml), the αvβ5-blocking mAb P1F6 (10 μg/ml), the α5-blocking mAb IIA1 (2 μg/ml) or the combinations of these mAbs. After 6 days of culturing, the mean tube length of duplicate wells was quantified (mm/cm2) as described (a). The data represent mean % ± SEM of FGF-2/TNF-α stimulated tube formation of 3–7 independent experiments. The mean tube length of FGF-2/TNF-α in the purified fibrin matrix was 83 ± 4 mm/cm2. b After 6 days of culturing, non-phase photomicrographs were taken of hMVECs stimulated with FGF-2/TNF-α in the absence or presence of LM609, IIA1 or the combination of these mAbs in the concentrations described above. Bar represents 300 μm. *P < 0.01 as compared to 100% (=FGF-2/TNF-α stimulated condition)
Discussion
In this report we demonstrate the interplay of αvβ3-, αvβ5- and α5β1-integrins in the formation of capillary-like structures in a 3-D in vitro plasma-derived fibrinous matrix. In the 3D-fibrinous matrix, tube formation was only largely inhibited when integrin antagonists were used together, in particular those blocking the αvβ3- together with the α5β1-integrins. Accordingly, endostatin in combination with either the αvβ3-integrin inhibiting mAb LM609 or the αv-competing cyclic-RGD peptide induced a dramatic decrease of tube formation, while endostatin alone had only a minimal effect. These data suggest that integrin-mediated interactions of endothelial cells with a fibrinous matrix are required for, but also support survival of, capillary tube formation by these cells.
The fibrinous exudates formed in wounds and tumor stroma are considered as supporting matrices for angiogenesis [4, 44, 45]. Our data indicate that the formation of capillary-like tubular structures in vitro proceeds similarly in a fibrin matrix prepared from purified fibrinogen and in a fibrinous exudate matrix prepared from plasma. Therefore, the focus was on the fibrin-recognizing integrins αvβ3, αvβ5 and α5β1, which have been implicated in angiogenesis [15, 16, 21, 46, 47]. These fibrin-binding integrins were present on the endothelium of newly formed vessels in a recanalized mural thrombus and conform previous reports [48–50]. The RGD-integrin inhibiting agent echistatin completely inhibited the FGF-2/TNF-α-induced formation of capillary-like structures by hMVECs, indicating that RGD-containing integrins were essential for neovascularization [15, 16, 21, 46]. Furthermore, our data indicate that a complete inhibition of capillary-like tube formation requires the simultaneous inhibition of these integrins, in particular the αvβ3- and α5β1-integrins. These data compare well with those of integrin inhibiting antibodies on the reorganization of endothelial cells embedded in a three-dimensional fibrin matrix [51]. The mutual interaction of α5β1-integrin and αv-containing integrins, in particular αvβ3-integrin was underscored by using two unrelated types of inhibitors for the α5β1-integrin (mAb IIA1, endostatin) and the αvβ3-integrin (mAb LM609, cRGD), which make inhibitor-specific effects less likely. Several recent reports have pointed to the induction of apoptosis in endothelial cells as a cause of angiogenesis inhibition by ligands of the αvβ3-integrin, such as mAb LM609 and tumstatin [34]. Our in vitro data did not provide direct evidence of the occurrence of such mechanism in endothelial cells that were in contact with a fibrinous matrix. This suggests that the interaction of hMVECs with the fibrin matrix may provide signals into the cells that protect them from going into apoptosis, once αvβ3-integrin is blocked. In this context, it should be noted that the adhesive potential of endothelial cells to bind fibrin(ogen) depends on transglutaminase and FXIIIa [52]. Oligomerization of the αC-domains of fibrinogen by transglutaminase, stimulates their ability to bind αvβ3-, αvβ5-, and α5β1-integrins on endothelial cells. The oligomerization did not only increase cell adhesion, but also promoted integrin-dependent cell signaling via focal adhesion kinase (FAK) and extracellular signal regulated kinase (ERK) [53].
Our data suggest that integrin–fibrin interactions were dominant in the plasma-derived fibrinous matrix, because a similar effect was observed with purified fibrin matrices as with plasma-derived fibrinous matrices. This directs the attention to the RGD-sequences in fibrin, of which the RGD-sequence 572–574 in the Aα-chain of human fibrinogen plays a major role in the invasion of hMVECs in a fibrin matrix [54–56]. Interestingly, this RGD-sequence is absent in mouse fibrinogen (Fig. 5), while three additional RGD-sequences were present between position 272 and 286 in the murine Aα-chain (compare human and mouse Aα-chain in Fig. 5). Thiagarajan et al. [54] have demonstrated that in the bovine fibrinogen the RGD-sequence at position 272–274, which is homologous to an RGG in human fibrinogen, can mediate endothelial cell adhesion. It is likely that this substitution plays a similar role in the murine fibrinogen. However, given the importance of the flanking regions in the specificity of the RGD-sequences data from mouse and man must be compared with cautiousness. Furthermore, we can not exclude an indirect additional role for integrin α3β1, which was reported to affect endothelial cell adhesion [57] and migration [58]. However, such effect will be limited, as inhibition of β1-integrins alone did not significantly alter endothelial tube formation in a fibrinous matrix.
In the fibrinous matrix, the inhibitory effect of endostatin is highly compatible with α5β1-integrin blocking activity. Although endostatin does not contain an RGD-sequence, it can interact with RGD-binding integrins, particularly α5β1-integrin [33], similar to the binding of the C-terminus of MMP-2 to RGD-dependent integrins [59].
Our in vitro data demonstrate that blocking of only one integrin is not sufficient for complete inhibition of tube formation by human MVEC in a human fibrin or plasma clot, but requires the simultaneous inhibition of αvβ3- and α5β1-integrins. Our data, as well as the recent findings of Carnevale et al. [60], support the use of a combined administration of αvβ3- and α5β1-integrin antagonists to block angiogenesis in an experimental setting. They showed that blocking of both β1- and β3-integrins resulted in inhibition of tube formation in fibrin matrices using the rat aortic ring model, whereas blocking of only one of the integrins was ineffective. Using collagen matrices they found that β1-integrins, but not β3-integrins were required for angiogenic sprouting. These data indicate that the integrin requirements may vary for neovessel formation, dependent on the composition of the ECM. Indeed, endothelial cells overexpress αvβ3-integrin when exposed to fibrin and during wound healing in a fibrin rich matrix, vascular cells transiently express αvβ3-integrin [26, 61]. Moreover, Bayless et al. [62] reported that the integrin-dependent spingosine-1-phosphate regulates endothelial cell invasion, lumen formation, and branching morphogenesis in fibrin matrices, which is dependent on both αvβ3- and α5β1-integrins.
Although the involvement of particular integrin combinations, essential for angiogenesis, remains controversial, it is likely that multiple integrin attack prevents compensatory mechanisms. And our finding demonstrates the flexibility of the angiogenesis process in a temporary fibrin matrix. Moreover, it also may bear impact on the use of inhibitors of specific integrins for anti-angiogenesis treatment [63, 64]. After initial damage of the blood vessel by blocking agents of individual integrins, the formation of a fibrinous exudate may support survival of endothelial cells.
References
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660. doi:10.1038/nm0603-653
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31. doi:10.1038/nm0195-27
Dvorak HF (1986) Tumors: wounds that do not heal similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
van Hinsbergh VWM, Collen A, Koolwijk P (2001) Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci 936:426–437
Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20:4368–4380. doi:10.1200/JCO.2002.10.088
Daly ME, Makris A, Reed M, Lewis CE (2003) Hemostatic regulators of tumor angiogenesis: a source of antiangiogenic agents for cancer treatment? J Natl Cancer Inst 95:1660–1673
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Clark RA (2001) Fibrin and wound healing. Ann N Y Acad Sci 936:355–367
Pepper MS (2001) Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol 21:1104–1117. doi:10.1161/hq0701.093685
Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD (1990) Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol 111:743–755. doi:10.1083/jcb.111.2.743
Koolwijk P, van Erck MG, de Vree WJ, Vermeer MA, Weich HA, Hanemaaijer R, van Hinsbergh VWM (1996) Cooperative effect of TNFα, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J Cell Biol 132:1177–1188. doi:10.1083/jcb.132.6.1177
Hotary KB, Yana I, Sabeh F, Li XY, Holmbeck K, Birkedal-Hansen H, Allen ED, Hiraoka N, Weiss SJ (2002) Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med 195:295–308. doi:10.1084/jem.20010815
Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR (2002) Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115:3427–3438
Collen A, Hanemaaijer R, Lupu F, Quax PH, van Lent N, Grimbergen J, Peters E, Koolwijk P, van Hinsbergh VWM (2003) Membrane-type matrix metalloproteinase-mediated angiogenesis in a fibrin-collagen matrix. Blood 101:1810–1817. doi:10.1182/blood-2002-05-1593
Rupp PA, Little CD (2001) Integrins in vascular development. Circ Res 89:566–572. doi:10.1161/hh1901.097747
Eliceiri BP, Cheresh DA (1999) The role of αv integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest 103:1227–1230. doi:10.1172/JCI6869
Eliceiri BP (2001) Integrin and growth factor receptor crosstalk. Circ Res 89:1104–1110. doi:10.1161/hh2401.101084
Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE (2008) Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol 28:1703–1713. doi:10.1161/ATVBAHA.108.172015
Hodivala-Dilke KM, Reynolds AR, Reynolds LE (2003) Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res 314:131–144. doi:10.1007/s00441-003-0774-5
Dejana E, Lampugnani MG, Giorgi M, Gaboli M, Marchisio PC (1990) Fibrinogen induces endothelial cell adhesion and spreading via the release of endogenous matrix proteins and the recruitment of more than one integrin receptor. Blood 75:1509–1517
Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA (1994) Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79:1157–1164. doi:10.1016/0092-8674(94)90007-8
Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA (1995) Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 96:1815–1822. doi:10.1172/JCI118227
Kim S, Bell K, Mousa SA, Varner JA (2000) Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol 156:1345–1362
Collo G, Pepper MS (1999) Endothelial cell integrin α5β1 expression is modulated by cytokines and during migration in vitro. J Cell Sci 112(Pt 4):569–578
Kumar CC, Malkowski M, Yin Z, Tanghetti E, Yaremko B, Nechuta T, Varner J, Liu M, Smith EM, Neustadt B, Presta M, Armstrong L (2001) Inhibition of angiogenesis and tumor growth by SCH221153, a dual αvβ3 and αvβ5 integrin receptor antagonist. Cancer Res 61:2232–2238
Feng X, Clark RA, Galanakis D, Tonnesen MG (1999) Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: stabilization of αv/β3 mRNA by fibrin1. J Invest Dermatol 113:913–919. doi:10.1046/j.1523-1747.1999.00786.x
Kroon ME, Koolwijk P, van der Vecht B, van Hinsbergh VWM (2000) Urokinase receptor expression on human microvascular endothelial cells is increased by hypoxia: implications for capillary-like tube formation in a fibrin matrix. Blood 96:2775–2783
Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM (2002) Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med 8:27–34. doi:10.1038/nm0102-27
Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM (2009) Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med 15:392–400. doi:10.1038/nm.1941
Hynes RO (2002) A reevaluation of integrins as regulators of angiogenesis. Nat Med 8:918–921. doi:10.1038/nm0902-918
Carmeliet P (2002) Integrin indecision. Nat Med 8:14–16. doi:10.1038/nm0102-14
Stupack DG, Cheresh DA (2002) Get a ligand, get a life: integrins, signalling and cell survival. J Cell Sci 115:3729–3738. doi:10.1242/jcs.00071
Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K (2001) Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci USA 98:1024–1029. doi:10.1073/pnas.031564998
Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R (2003) Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc Natl Acad Sci USA 100:4766–4771. doi:10.1073/pnas.0730882100
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88:277–285. doi:10.1016/S0092-8674(00)81848-6
Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR (1999) Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J 18:4414–4423. doi:10.1093/emboj/18.16.4414
Dixelius J, Cross M, Matsumoto T, Sasaki T, Timpl R, Claesson-Welsh L (2002) Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res 62:1944–1947
Kroon ME, Koolwijk P, van Goor H, Weidle UH, Collen A, van der Pluijm G, van Hinsbergh VWM (1999) Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol 154:1731–1742
Defilippi P, van Hinsbergh VWM, Bertolotto A, Rossino P, Silengo L, Tarone G (1991) Differential distribution and modulation of expression of α1/β1 integrin on human endothelial cells. J Cell Biol 114:855–863. doi:10.1083/jcb.114.4.855
van Hinsbergh VWM, Sprengers ED, Kooistra T (1987) Effect of thrombin on the production of plasminogen activators and PA inhibitor-1 by human foreskin microvascular endothelial cells. Thromb Haemost 57:148–153
Engelse MA, Laurens N, Verloop RE, Koolwijk P, van Hinsbergh VWM (2008) Differential gene expression analysis of tubule forming and non-tubule forming endothelial cells: CDC42GAP as a counter-regulator in tubule formation. Angiogenesis 11:153–167. doi:10.1007/s10456-007-9086-9
van Boheemen PA, van den Hoogen CM, Koolwijk P (1995) Comparison of the inhibition of urokinase-type plasminogen activator (u-PA) activity by monoclonal antibodies specific for u-PA as assessed by different assays. Fibrinolysis 9:343–349. doi:10.1016/S0268-9499(95)80081-6
Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT (1996) Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med 2:529–533. doi:10.1038/nm0596-529
Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM (1987) Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 57:673–686
Polverini PJ (1996) How the extracellular matrix and macrophages contribute to angiogenesis-dependent diseases. Eur J Cancer 32A:2430–2437. doi:10.1016/S0959-8049(96)00386-3
Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA (1995) Definition of two angiogenic pathways by distinct alpha v integrins. Science 270:1500–1502. doi:10.1126/science.270.5241.1500
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin αvβ3 for angiogenesis. Science 264:569–571. doi:10.1126/science.7512751
Klein S, Giancotti FG, Presta M, Albelda SM, Buck CA, Rifkin DB (1993) Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell 4:973–982
Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715. doi:10.1146/annurev.cellbio.12.1.697
Ruoslahti E, Engvall E (1997) Integrins and vascular extracellular matrix assembly. J Clin Invest 99:1149–1152. doi:10.1172/JCI119269
Bayless KJ, Salazar R, Davis GE (2000) RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol 156:1673–1683
Dallabrida SM, Falls LA, Farrell DH (2000) Factor XIIIa supports microvascular endothelial cell adhesion and inhibits capillary tube formation in fibrin. Blood 95:2586–2592
Belkin AM, Tsurupa G, Zemskov E, Veklich Y, Weisel JW, Medved L (2005) Transglutaminase-mediated oligomerization of the fibrin(ogen) αC domains promotes integrin-dependent cell adhesion and signaling. Blood 105:3561–3568. doi:10.1182/blood-2004-10-4089
Thiagarajan P, Rippon AJ, Farrell DH (1996) Alternative adhesion sites in human fibrinogen for vascular endothelial cells. Biochemistry 35:4169–4175. doi:10.1021/bi952532b
Collen A, Maas A, Kooistra T, Lupu F, Grimbergen J, Haas FJ, Biesma DH, Koolwijk P, Koopman J, van Hinsbergh VWM (2001) Aberrant fibrin formation and cross-linking of fibrinogen Nieuwegein, a variant with a shortened Aα-chain, alters endothelial capillary tube formation. Blood 97:973–980. doi:10.1182/blood.V97.4.973
Kaijzel EL, Koolwijk P, van Erck MG, van Hinsbergh VWM, de Maat MP (2006) Molecular weight fibrinogen variants determine angiogenesis rate in a fibrin matrix in vitro and in vivo. J Thromb Haemost 4:1975–1981. doi:10.1111/j.1538-7836.2006.02081.x
Hutchings H, Ortega N, Plouet J (2003) Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J 17:1520–1522
Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W (2004) Cross-talk of integrin alpha3beta1 and tissue factor in cell migration. Mol Biol Cell 15:4416–4425. doi:10.1091/mbc.E03-09-0640
Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell 85:683–693. doi:10.1016/S0092-8674(00)81235-0
Carnevale E, Fogel E, Aplin AC, Gelati M, Howson KM, Zhu WH, Nicosia RF (2007) Regulation of postangiogenic neovessel survival by β1 and β3 integrins in collagen and fibrin matrices. J Vasc Res 44:40–50. doi:10.1159/000097976
Clark RA, Tonnesen MG, Gailit J, Cheresh DA (1996) Transient functional expression of alphaVbeta 3 on vascular cells during wound repair. Am J Pathol 148:1407–1421
Bayless KJ, Davis GE (2003) Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun 312:903–913. doi:10.1016/j.bbrc.2003.11.017
Mundhenke C, Thomas JP, Wilding G, Lee FT, Kelzc F, Chappell R, Neider R, Sebree LA, Friedl A (2001) Tissue examination to monitor antiangiogenic therapy: a phase I clinical trial with endostatin. Clin Cancer Res 7:3366–3374
Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA (2000) Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin αvβ3. Clin Cancer Res 6:3056–3061
Acknowledgments
This study was supported by the Netherlands Organization of Scientific Research—Medical Sciences (grant 902-17-090) and STW/DPTE (grants BGT.6733 and BGT.7647)
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Laurens, N., Engelse, M.A., Jungerius, C. et al. Single and combined effects of αvβ3- and α5β1-integrins on capillary tube formation in a human fibrinous matrix. Angiogenesis 12, 275–285 (2009). https://doi.org/10.1007/s10456-009-9150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-009-9150-8