Abstract
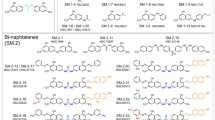
The protein processing enzyme, methionine aminopeptidase-2 (MetAP-2), has been identified as a molecular target of fumagillin and its derivative, TNP-470, compounds known to inhibit endothelial cell proliferation and angiogenesis. A high-throughput screening program was undertaken to identify selective, reversible inhibitors of MetAP-2 in an attempt to discover structurally novel anti-angiogenic agents for potential therapeutic use in oncology. Approximately 90 small-molecule, reversible, selective inhibitors of rhMetAP-2 were identified. The most potent of these compounds contained a singly-substituted triazole moiety which exhibited an IC50 of 8 nM (95% confidence limits 5 to 13 nM) and was highly selective for MetAP-2 over MetAP-1 ( 60-fold difference in IC50 values). Unlike fumagillin, these MetAP-2 inhibitors failed to significantly inhibit growth factor-stimulated endothelial cell (HUVEC) proliferation or to suppress angiogenesis in the in vitroaortic ring explant model of microvessel outgrowth. The MetAP-2-inhibitory activity of these compounds was dependent on the divalent cation used as the metalloenzyme activating cofactor for MetAP-2. These inhibitors were identified using cobalt(II)-activated recombinant human MetAP-2 for screening compound libraries. When manganese (Mn2+) was substituted for cobalt following EDTA treatment and extensive dialysis of the MetAP-2 protein, these inhibitors were significantly less potent (40-fold increase in IC50) as inhibitors of MetAP-2. These results support the recent hypothesis that cobalt may not be the relevant divalent metal ion cofactor for MetAP-2 in cells and may explain the observed absence of cell-based activity using potent triazole inhibitors of cobalt-activated MetAP-2
Similar content being viewed by others
References
Bradshaw RA, Brickey WW, Walker KW. N-terminal processing: The methionine aminopeptidase and N alpha-acetyl transferase families. Trends Biochem Sci 1998; 23 (7): 263–7.
Wang J, Lou P, Henkin J. Selective inhibition of endothelial cell proliferation by fumagillin is not due to differential expression of methionine aminopeptidases. J Cell Biochem 2000; 77 (3): 465–73.
Sin N, Meng L, Wang MQ et al. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA 1997; 94 (12): 6099–103.
Griffith EC, Su Z, Turk BE et al. Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol 1997; 4 (6): 461–71.
Griffith EC, Su Z, Niwayama S et al. Molecular recognition of angiogenesis inhibitors fumagillin and ovalicin by methionine aminopeptidase 2. Proc Natl Acad Sci USA 1998; 95(26): 15183–8.
Kusaka M, Sudo K, Fujita T et al. Potent anti-angiogenic action of AGM-1470: Comparison to the fumagillin parent. Biochem Biophys Res Commun 1991; 174 (3): 1070–6.
Yeh JR, Mohan R, Crews CM. The antiangiogenic agent TNP-470 requires p53 and p21CIP/WAF for endothelial cell growth arrest. Proc Natl Acad Sci USA 2000; 97 (23): 12782–7.
Zhang Y, Griffith EC, Sage J, et al. Cell cycle inhibition by the antiangiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc Natl Acad Sci USA 2000; 97 (12): 6427–32.
Kusaka M, Sudo K, Matsutani E et al. Cytostatic inhibition of endothelial cell growth by the angiogenesis inhibitor TNP-470 (AGM-1470). Br J Cancer 1994; 69 (2): 212–6.
Bhargava P, Marshall JL, Rizvi N et al. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin Cancer Res 1999; 5 (8): 1989–95.
Wang J, Quan N, Henkin J. Human endothelial cells are exceptionally sensitive to loss of methionine aminopeptidase-2 (MetAP-2). Proc Am Assoc Cancer Res 1998; 39: 98.
Phillips PE, Towbin H, Stolz B et al. A novel biomarker for methionine aminopeptidase inhibitors: NH2-terminal changes in 14–3–3c and detection in in vitro and in vivo samples treated with NVP-LAF389. Proc Am Assoc Cancer Res 2002; 43: 4732 [abstract].
Towbin H, Bair KW, DeCaprio JA et al. Proteomics-based target identification: bengamides as a new class of methionine aminopeptidase inhibitors. J Biol Chem 2003; 278 (52): 52964–71.
Li X, Chang YH. Evidence that the human homologue of a rat initiation factor-2 associated protein (p67) is a methionine aminopeptidase. Biochem Biophys Res Commun 1996; 227 (1): 152–9.
Ratner S. L-Amino Acid Oxidases. In Colowick SP, Caplan NO (eds): Mammalian Tissues and Snake Venom. Methods in Enzymology. New York: Academic Press 1955; 204–11.
Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989; 96 (3): 736–49.
Muir D, Varon S, Manthorpe M. An enzyme-linked immunosorbent assay for bromodeoxyuridine incorporation using fixed microcultures. Anal Biochem 1990; 185 (2): 377–82.
Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest 1990; 63 (1): 115–22.
Nissanov J, Tuman RW, Gruver LM, Fortunato JM. Automatic vessel segmentation and quantification of the rat aortic ring assay of angiogenesis. Lab Invest 1995; 73 (5): 734–9.
Wang J, Sheppard GS, Lou P et al. Physiologically relevant metal cofactor for methionine aminopeptidase-2 is manganese. Biochemistry 2003; 42 (17): 5035–42.
Roderick SL, Matthews BW. Structure of the cobalt-dependent methionine aminopeptidase from Escherichia coli: A new type of proteolytic enzyme. Biochemistry 1993; 32(15): 3907–12.
Liu S, Widom J, Kemp CW Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science 1998; 282 (5392): 1324–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garrabrant, T., Tuman, R., Ludovici, D. et al. Small molecule inhibitors of methionine aminopeptidase type 2 (MetAP-2) fail to inhibit endothelial cell proliferation or formation of microvessels from rat aortic rings in vitro. Angiogenesis 7, 91–96 (2004). https://doi.org/10.1007/s10456-004-6089-7
Issue Date:
DOI: https://doi.org/10.1007/s10456-004-6089-7