Abstract
Background
The expression of fungal allergens is increased by the germination of conidia. We assessed the state of germination of fungal conidia recovered by nasal lavage after environmental exposure.
Methods
Nasal lavage was performed on twenty adults at three stages: the start of the experiment, after 1 h indoors, and after 1 h outdoors. One half of the lavage liquid was immediately treated to prevent in-vitro germination and stained with periodic acid Schiff (PAS) to enable identification of germinated and ungerminated conidia. The untreated half of the lavage liquid was cultured on nutrient agar plates to enumerate and identify viable fungi.
Results
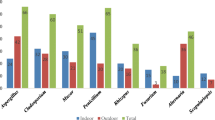
PAS staining showed that both ungerminated and germinated conidia, and hyphal fragments, were present in the nasal cavity. The most prevalent fungi recovered were Aspergillus, Alternaria, Cladosporium, Epicoccum, Penicillium, and Yeast species. The number of viable fungi recovered after 1 h indoors was significantly less than after 1 h outdoors (P < 0.01).
Conclusions
Viable fungi and germinating conidia, in addition to ungerminated conidia and hyphal fragments, were present in the nasal cavity after both indoor and outdoor exposure. This provides novel insight into the pathogenicity of exposure to fungal aeroallergens.


Similar content being viewed by others
Abbreviations
- PAS:
-
Periodic acid Schiff
- V8:
-
20% Vegetable juice nutrient agar
- RB:
-
Rose-Bengal chloramphenicol nutrient agar
References
Andersson, M., Downs, S. H., Mitakakis, T. Z., Leuppi, J. D., & Marks, G. B. (2003). Natural exposure to Alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatric Allergy and Immunology, 14(2), 100–105.
Braun, H., Stammberger, H., Buzina, W., Freudenschuss, K., Lackner, A., & Beham, A. (2003). Incidence and detection of fungi and eosinophilic granulocytes in chronic rhinosinusitis. Laryngo Rhino Otologie, 82(5), 330–340.
Burge, H. A., Pierson, D. L., Groves, T. O., Strawn, K. F., & Mishra S. K. (2000). Dynamics of airborne fungal populations in a large office building. Current Microbiology, 40(1), 10–16.
Burge, H. P., Solomon, W. R., & Boise, J. R. (1977). Comparative merits of eight popular media in aerometric studies of fungi. Journal of Allergy and Clinical Immunology, 60(3), 199–203.
Chew, G. L., Rogers, C., Burge, H. A., Muilenberg, M. L., & Gold, D. R. (2003). Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy, 58(1), 13–20.
Cocks, T. M., & Moffatt, J. D. (2001). Protease-activated receptor-2 (PAR-2) in the airways. Pulmonary Pharmacology and Therapeutics, 14(3), 183–191.
Cramer, R. A., & Lawrence, C. B. (2003). Cloning of a gene encoding an alt a 1 isoallergen differentially expressed by the necrotrophic fungus Alternaria brassicicola during arabidopsis infection. Applied and Environmental Microbiology, 69(4), 2361–2364.
Dennis, D. P. (2003). Chronic sinusitis: Defective t-cells responding to superantigens, treated by reduction of fungi in the nose and air. Archives of Environmental Health, 58(7), 433–441.
Downs, S. H., Mitakakis, T. Z., Marks, G. B., Car, N. G., Belousova, E. G., Leuppi, J. D., Xuan, W., Downie, S. R., Tobias, A., & Peat, J. K. (2001). Clinical importance of Alternaria exposure in children. American Journal of Respiratory and Critical Care Medicine, 164(3), 455–459.
Green, B. J., Mitakakis, T. Z., & Tovey, E. R. (2003). Allergen detection from 11 fungal species before and after germination. Journal of Allergy and Clinical Immunology, 111(2), 285–289.
Green B. J., Tovey E. R., Sercombe J. K., Blachere F. M., Beezhold D. H., & Schmechel D. (2006). Airborne fungal fragments and allergenicity, Medical Mycology, 44(Suppl. 1), S245–S255.
Greiff, L., Pipkorn, U., Alkner, U., & Persson, C.G. (1990). The ‘nasal pool’ device applies controlled concentrations of solutes on human nasal airway mucosa and samples its surface exudations/secretions. Clinical and Experimental Allergy, 20(3), 253–259.
Karpovich-Tate, N., Dewey, F. M., Smith, E. J., Lund V. J., Gurr P. A., & Gurr, S. J. (2000). Detection of fungi in sinus fluid of patients with allergic fungal rhinosinusitis. Acta Oto-Laryngology, 120(2), 296–302.
Kauffman, H. F., Tomee, J. F., van de Riet, M. A., Timmerman, A. J., & Borger, P. (2000). Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. Journal of Allergy and Clinical Immunology, 105(6 Pt 1), 1185–1193.
Kauffman, H. F. (2003). Interaction of environmental allergens with airway epithelium as a key component of asthma. Current Allergy and Asthma Reports, 3(2), 101–108.
Keck, T., Leiacker, R., Riechelmann, H., & Rettinger, G. (2000). Temperature profile in the nasal cavity. Laryngoscope, 110(4), 651–654.
Knutsen, A. P., Bellone, C., & Kauffman, H. (2002). Immunopathogenesis of allergic bronchopulmonary aspergillosis in cystic fibrosis. Journal of Cystic Fibrosis, 1(2), 76–89.
Lackner A., Freudenschuss K., Buzina W., Stammberger H., Panzitt T., Schosteritsch S., & Braun H. (2004). From when on can fungi be identified in nasal mucus of humans? Laryngo Rhino Otologie, 83(2), 117–121.
Lake, F. R., Froudist, J. H., McAleer, R., Gillon, R. L., Tribe, A. E., & Thompson, P. J. (1991). Allergic bronchopulmonary fungal disease caused by Bipolaris and Curvularia. Australian and New Zealand Journal of Medicine, 21, 871–874.
McManus, J. F. A. (1946). Histological demonstration of mucin after periodic acid. Nature, 158(4006), 202–202
Meletiadis, J., Meis, J. F., Mouton, J. W., & Verweij, P. E. (2001). Analysis of growth characteristics of filamentous fungi in different nutrient media. Journal of Clinical Microbiology, 39(2), 478–484.
Mitakakis, T. Z., Barnes, C., & Tovey, E. R. (2001). Spore germination increases allergen release from Alternaria. Journal of Allergy and Clinical Immunology, 107(2), 388–390.
Passone, M. A., Resnik, S. L., & Etcheverry, M. G. (2005). In vitro effect of phenolic antioxidants on germination, growth and aflatoxin b accumulation by peanut Aspergillus section flavi. Journal of Applied Microbiology, 99(3), 682–691.
Ponikau, J., Sherris, D., Kern, E., Homburger, H., Frigas, E., Gaffey, T., & Roberts, G. (1999). The diagnosis and incidence of allergic fungal sinusitis. Mayo Clinic Proceedings, 74(9), 877–884.
Ponikau, J. U., Sherris, D.A., Kita, H., Kern, E. B. (2002). Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. Journal of Allergy and Clinical Immunology, 110(6), 862–866.
Proctor, D. F., Andersen, I., & Lundqvis, G. (1973). Clearance of inhaled particles from human nose. Archives of Internal Medicine, 131(1), 132–139.
Reed, C. E., & Kita, H. (2004). The role of protease activation of inflammation in allergic respiratory diseases (review). Journal of Allergy and Clinical Immunology, 114(5), 997–1008.
Robinson, B. W., Venaille, T. J., Mendis, A. H., & McAleer, R. (1990). Allergens as proteases: An Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. Journal of Allergy and Clinical Immunology, 86(5), 726–731.
Schubert, M. S., Hutcheson, P. S., Graff, R. J., Santiago L., & Slavin R.G. (2004) Hla-dqb1 *03 in allergic fungal sinusitis and other chronic hypertrophic rhinosinusitis disorders. Journal of Allergy and Clinical Immunology, 114(6), 1376–1383.
Sporik, R. B., Arruda, L. K., Woodfolk, J., & Chapman, M. D., Platts-Mills, T. A. (1993). Environmental exposure to Aspergillus fumigatus allergen (Asp f 1). Clinical and Experimental Allergy, 23(4), 326–331.
Su, W., Liu, C., Hung, S., & Tsai, W. (1983). Bacteriological study in chronic maxillary sinusitis. Laryngoscope, 93(7), 931–934.
Tafaghodi, M., Abolghasem Sajadi Tabassi, S., Jaafari, M. R., Zakavi, S. R., & Momen-Nejad, M. (2004). Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. International Journal of Pharmaceutics, 280(1–2), 125–135.
Weschta, M., Rimek, D., Formanek, M., Polzehl, D., Podbielski, A., & Riechelmann, H. (2004). Topical antifungal treatment of chronic rhinosinusitis with nasal polyps: A randomised, double-blind clinical trial. Journal of Allergy and Clinical Immunology, 113(6), 1122–1128.
Yang, Z., Jaeckisch, S. M., & Mitchell, C. G. (2000). Enhanced binding of Aspergillus fumigatus spores to a549 epithelial cells and extracellular matrix proteins by a component from the spore surface and inhibition by rat lung lavage fluid. Thorax, 55(7), 579–584.
Acknowledgements
This research was supported by a grant from the National Health and Medical Research Council, Australia (Grant Number 253818). Volunteer participation was approved by the University of Sydney Human Research Ethics Committee (Approval Number 3577). Facilities for PAS staining were kindly provided by the Histopathology Laboratory, Department of Pathology, The University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sercombe, J.K., Green, B.J. & Tovey, E.R. Recovery of germinating fungal conidia from the nasal cavity after environmental exposure. Aerobiologia 22, 295–304 (2006). https://doi.org/10.1007/s10453-006-9043-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-006-9043-x